Abstract
Introduction
Ground glass opacity featured lung adenocarcinomas (GGO-LUAD) display more indolent biological behavior than solid nodule featured lung adenocarcinomas (SN-LUAD) and have an excellent prognosis. However, the cellular immune characteristics of GGO-LUAD remain poorly understood.
Methods
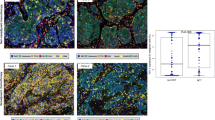
Immunohistochemistry technique was performed to stain related immune markers (CD8, CD103, CD20, CD138, CD4, FOXP3, CD68, CD163, PD-1 and PD-L1) and TGF-β from 15 patients with pure GGO-LUAD and 15 patients with SN-LUAD tissue sections (Paired cohort), and then, the related markers with significant differences were verified on 10 patients (Verified cohort) with both pure GGO-LUAD and SN-LUAD. For localization analysis of CD68 + tumor-associated macrophages (TAMs) and FOXP3 + Terg cells in tumor areas, pure GGO-LUAD and SN-LUAD were also stained for simultaneous detection of pan-CK, CD68 and FOXP3 by multiplex immunofluorescence.
Results
In the Paired cohort, compared with SN-LUAD, only the infiltration of TAMs and Treg cells was significantly lower in GGO-LUAD. The infiltration of the remaining immune cells including CD8 + T cells, CD4 + T cells, CD103 + T cells, CD20 + B cells and CD138 + Plasma cells in GGO-LUAD, although relatively low, was not significantly different. Meanwhile, the expression of TGF-β was significantly higher in SN-LUAD. And the above results have also been confirmed in the Verified cohort. Moreover, there was no significantly difference in PD-L1 expression in GGO-LUAD compared to SN-LUAD both in the Paired cohort and Verified cohort.
Conclusions
GGO-LUAD demonstrates an overall less active immune landscape as compared with SN-LUAD. TAMs and TGF-β may play an important role in the progression of GGO-LUAD. More importantly, PD-L1 expression in GGO-LUAD is comparable to that in SN-LUAD, indicating that there may be other reasons for the insensitivity of GGO-LUAD to immunotherapy.








Similar content being viewed by others
Data availability
The dataset used for this study is available from the corresponding author upon reasonable request.
Abbreviations
- GGO-LUAD:
-
Ground glass opacity featured lung adenocarcinomas
- SN-LUAD:
-
Solid nodule featured lung adenocarcinomas
- TAMs:
-
Tumor-associated macrophages
- LDCT:
-
Low-dose computed tomography
- HRCT:
-
High-resolution computed tomography
- FFPE:
-
Formalin-fixed, paraffin-embedded
- OS:
-
Overall survival
- AAH:
-
Atypical adenomatous hyperplasia
- AIS:
-
Adenocarcinoma in situ
- MIA:
-
Minimally invasive adenocarcinoma
- IAC:
-
Invasive lung adenocarcinoma
- TME:
-
The tumor microenvironment
References
Akhurst RJ, Hata A (2012) Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11:790–811
Allinson JP, Brown J, Gibb K, Navani N (2019) Immunotherapy in non-small cell lung cancer. Which patients and at which stage? Am J Respir Crit Care Med 199:1277–1279
Altorki NK, Borczuk AC, Harrison S et al (2022) Global evolution of the tumor microenvironment associated with progression from preinvasive invasive to invasive human lung adenocarcinoma. Cell Rep 39:110639
Amin MB, Edge S, Greene F et al (2017) AJCC cancer staging manual, 8th edn. Springer, pp 431–456
Chen H, Carrot-Zhang J, Zhao Y et al (2019) Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun 10:5472
Chen J, Tan Y, Sun F et al (2020) Single-cell transcriptome and antigen-immunoglobin analysis reveals the diversity of B cells in non-small cell lung cancer. Genome Biol 21:152
Chen K, Bai J, Reuben A et al (2021) Multiomics analysis reveals distinct immunogenomic features of lung cancer with ground glass opacity. Am J Respir Crit Care Med 204:1180–1192
Cooper WA, Tran T, Vilain RE et al (2015) PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 89:181–188
Dejima H, Hu X, Chen R et al (2021) Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features. Nat Commun 12:2722
Flavell RA, Sanjabi S, Wrzesinski SH et al (2010) The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 10:554–567
Forde PM, Chaft JE, Smith KN et al (2018) Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 378:1976–1986
Fu F, Zhang Y, Wen Z et al (2019) Distinct prognostic factors in patients with stage I non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol 14:2133–2142
Ganesan A-P, Clarke J, Wood O et al (2017) Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol 18:940–950
Guo X, Zhang Y, Zheng L et al (2018) Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med 24:978–985
Hattori A, Hirayama S, Matsunaga T et al (2019) Distinct clinicopathologic characteristics and prognosis based on the presence of ground glass opacity component in clinical stage IA lung adenocarcinoma. J Thorac Oncol 14:265–275
Hattori A, Takamochi K, Oh S et al (2020) Prognostic classification of multiple primary lung cancers based on a ground-glass opacity component. Ann Thorac Surg 109:420–427
Hu X, Fujimoto J, Ying L et al (2019) Multi-region exome sequencing reveals genomic evolution from preneoplasia to lung adenocarcinoma. Nat Commun 10:2978
Kim EY, Cha YJ, Lee SH et al (2022) Early lung carcinogenesis and tumor microenvironment observed by single-cell transcriptome analysis. Transl Oncol 15:101277
Krysan K, Tran LM, Grimes BS et al (2019) The immune contexture associates with the genomic landscape in lung adenomatous premalignancy. Cancer Res 79:5022–5033
Lu T, Yang X, Shi Y et al (2020) Single-cell transcriptome atlas of lung adenocarcinoma featured with ground glass nodules. Cell Discov 6:69
MacMahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284:228–243
Mantovani A, Marchesi F, Malesci A et al (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416
McWilliams A, Tammemagi MC, Mayo JR et al (2013) Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 369:910–919
Miyoshi T, Aokage K, Katsumata S et al (2019) Ground-glass opacity is a strong prognosticator for pathologic stage IA lung adenocarcinoma. Ann Thorac Surg 108:249–255
Naito M, Aokage K, Saruwatari K et al (2016) Microenvironmental changes in the progression from adenocarcinoma in situ to minimally invasive adenocarcinoma and invasive lepidic predominant adenocarcinoma of the lung. Lung Cancer 100:53–62
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Qian J, Zhao S, Zou Y et al (2020) Genomic underpinnings of tumor behavior in in situ and early lung adenocarcinoma. Am J Respir Crit Care Med 201:697–706
Quezada SA, Peggs KS, Simpson TR, Allison JP (2011) Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev 241:104–118
Rusch VW, Chaft JE, Johnson B et al (2018) Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): initial results from a multicenter study (LCMC3). JCO 36:8541
Santarpia M, González-Cao M, Viteri S et al (2015) Programmed cell death protein-1/programmed cell death ligand-1 pathway inhibition and predictive biomarkers: understanding transforming growth factor-beta role. Transl Lung Cancer Res 4:728–742
Sepesi B, Cascone T, William W et al (2019) Surgical outcomes following neoadjuvant nivolumab or nivolumab plus ipilimumab in non-small cell lung cancer—NEOSTAR Study. J Thorac Oncol 14:S241–S242
Sivakumar S, Lucas FAS, McDowell TL et al (2017) Genomic landscape of atypical adenomatous hyperplasia reveals divergent modes to lung adenocarcinoma. Cancer Res 77:6119–6130
Suda K, Shimoji M, Shimizu S et al (2019) Comparison of PD-L1 expression status between pure-solid versus part-solid lung adenocarcinomas. Biomolecules 9:456
Tao H, Mimura Y, Aoe K et al (2012) Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 75:95–101
Tauriello DVF, Palomo-Ponce S, Stork D et al (2018) Tgfbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554:538–543
Tauriello DVF, Sancho E, Batlle E (2022) Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer 22:25–44
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 6:244–285
Wang SS, Liu W, Ly D et al (2019) Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol 16:6–18
Wu F, Li W, Zhao W et al (2020) Synchronous ground-glass nodules showed limited response to anti-PD-1/PD-L1 therapy in patients with advanced lung adenocarcinoma. Clin Transl Med 10:e149
Xing X, Yang F, Huang Q et al (2021) Decoding the multicellular ecosystem of lung adenocarcinoma manifested as pulmonary subsolid nodules by single-cell RNA sequencing. Sci Adv 7:eabd9738
Yang L, Zhang Y (2017) Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol 10:58
Ye T, Deng L, Wang S et al (2019) Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol 14:617–627
Zeng C, Gao Y, Xiong J et al (2020) Tumor-infiltrating CD8+ T cells in ALK-positive lung cancer are functionally impaired despite the absence of PD-L1 on tumor cells. Lung Cancer 150:139–144
Zhang C, Zhang J, Xu FP et al (2019) Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. J Thorac Oncol 14:1912–1923
Zhang Y, Li G, Li Y et al (2020) Imaging features suggestive of multiple primary lung adenocarcinomas. Ann Surg Oncol 27:2061–2070
Zhang C, Yin K, Liu SY et al (2021) Multiomics analysis reveals a distinct response mechanism in multiple primary lung adenocarcinoma after neoadjuvant immunotherapy. J Immunother Cancer 9:e002312
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Acknowledgements
We thank all the contributing authors for their great effort on this article and greatly appreciate all patients who contributed to this study.
Funding
The study is funded by the Tongji Hospital Clinical Research Flagship Program (No.2019CR107). The funding sources had no influence on the analysis and interpretation of data or on the contents of the manuscript.
Author information
Authors and Affiliations
Contributions
RQ: conceptualization, data curation, formal analysis, software, investigation, methodology, supervision, visualization, roles/writing—original draft, writing—review and editing. FY: data curation, formal analysis, software, writing—review and editing. SH: data curation and investigation. BW: data curation and methodology. SQ: data curation, formal analysis, project administration and visualization. JX: data curation, project administration and visualization. XF: data curation, formal analysis, investigation, writing—review and editing. LL: data curation, formal analysis, investigation, validation, visualization, writing—review and editing. YC: Conceptualization, Data curation, Formal analysis, Investigation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Roles/Writing—original draft, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approvals were obtained by the institutional review board of Tongji Medical College of Huazhong University of Science and Technology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, R., Ye, F., Hu, S. et al. Distinct cellular immune profiles in lung adenocarcinoma manifesting as pure ground glass opacity versus solid nodules. J Cancer Res Clin Oncol 149, 3775–3788 (2023). https://doi.org/10.1007/s00432-022-04289-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04289-3