Abstract
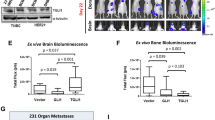
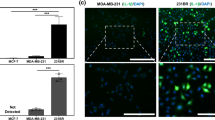
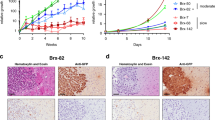
Treatment options for brain metastatic breast cancer are limited because the molecular mechanism for how breast cancer cells infiltrate the brain is not fully understood. For breast tumors to metastasize to the brain first, cells need to detach from the primary tumor, enter in the blood circulation, survive within the microvascular niche, and then cross the blood–brain barrier (BBB) to colonize into the brain. It is critical to understand how breast cancer cells transmigrate through the BBB to prevent brain metastasis. Nuclear respiratory factor 1 (NRF1) transcription factor has been reported to be highly active in several human cancers and its aberrant expression facilitates in the acquisition of breast cancer stem cells (BCSCs). Inhibitor of differentiation protein 3 (ID3), a transcription regulating protein, induces pluripotent endothelial stem cells (ESCs). Herein, we investigated if NRF1-induced BCSCs could cross a BBB model and guiding of BCSCs by ID3-induced ESCs across the BBB. BCSCs and ESCs were subjected to functional gain/loss experiments to determine if NRF1/ID3 contributed to lineage-specific BCSCs organ entry. First, we tested whether NRF1 promoted migration of breast cancer using a BBB model consisting of BCSCs or MDA-MB231 cells, brain endothelial cell layer, and astrocytes. NRF1 overexpression increased the propensity for BCSCs and NRF1-induced MDA-MB231 cells to adhere to brain endothelial cells and migrate across a human BBB model. Increased adhesion of NRF1-induced BCSCs to ESCsID3 was detected. NRF1-induced BCSCs crossed through the BBB model and this was promoted by ESCsID3. We also showed that environmental relevant exposure to PCBs (PCB153 and PCB77) produced differential effects. Treatment with PCB153 showed increased growth of NRF1-induced BCSCs tumor spheroids and increased in vivo migration of ESCsID3. Exosomal ID3 released from endothelial cells also supported the growth of NRF1-induced BCSCs and provide the basis for paracrine effects by ESCsID3 associated with breast tumors. Xenograft experiments showed that ID3 overexpressing brain ESCs not only supported the growth of BCSC tumor spheroids but guided them to the neural crest in zebrafish. These findings show for the first time a novel role for ID3 and NRF1 by which ESCsID3 help guide BCSCsNRF1 to distant metastatic sites where they most likely facilitate the colonization, survival, and proliferation of BCSCs. This knowledge is important for pre-clinical testing of NRF1/ID3 modifying agents to prevent the spread of breast cancer to the brain.









Similar content being viewed by others
References
Barakat R, Oakley O, Kim H, Jin J, Ko CMJ (2016) Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep 49(9):488–496
Brugnoli F, Grassilli S, Piazzi M et al (2013) In triple negative breast tumor cells, PLC-β2 promotes the conversion of CD133high to CD133low phenotype and reduces the CD133-related invasiveness. Mol Cancer 12:165. https://doi.org/10.1186/1476-4598-12-165
Chen YH, Wu ZQ, Zhao YL, Si YL, Guo MZ, Han WD (2012) FHL2 inhibits the Id3-promoted proliferation and invasive growth of human MCF-7 breast cancer cells. Chin Med J (Engl) 125:2329–2333
Chu S, Covaci A, Schepens P (2003) Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ Res 93(2):167–176
Das JK, Felty Q (2014) PCB153-induced overexpression of ID3 contributes to the development of microvascular lesions. PLoS ONE 9(8):e104159. https://doi.org/10.1371/journal.pone.0104159 (PMID: 25090023, PMCID: PMC4121297)
Das JK, Felty Q (2015) Microvascular lesions by estrogen-induced ID3: its implications in cerebral and cardiorenal vascular disease. J Mol Neurosci. 55(3):618–631. https://doi.org/10.1007/s12031-014-0401-9 (Epub 2014 Aug 17. PMID: 25129100, PMCID: PMC4320014)
Das JK, Voelkel NF, Felty Q (2015) ID3 contributes to the acquisition of molecular stem cell-like signature in microvascular endothelial cells: its implication for understanding microvascular diseases. Microvasc Res 98:126–138. https://doi.org/10.1016/j.mvr.2015.01.006
Das JK, Felty Q, Poppiti R, Jackson RM, Roy D (2018) Nuclear respiratory factor 1 acting as an oncoprotein drives estrogen-induced breast carcinogenesis. Cells 7(12):234. https://doi.org/10.3390/cells7120234
Felty Q, Porther N (2008) Estrogen-induced redox sensitive Id3 signaling controls the growth of vascular cells. Atherosclerosis 198(1):12–21. https://doi.org/10.1016/j.atherosclerosis.2007.12.048 (Epub 2008 Feb 20 PMID: 18281048)
Ghajar CM, Peinado H, Mori H et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15(7):807–817. https://doi.org/10.1038/ncb2767
Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, TodorovaManova K, Gerald WL, Brogi E, Benezra R, Massagué J (2007) ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci USA 104:19506–19511
Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW, Pennell KD (2012) Association between polychlorinated biphenyls and Parkinson’s disease neuropathology. Neurotoxicology 33(5):1298–1304
Isozaki T, Amin MA, Arbab AS et al (2014) Inhibitor of DNA binding 1 as a secreted angiogenic transcription factor in rheumatoid arthritis. Arthritis Res Ther 16:R68. https://doi.org/10.1186/ar4507
Li F, Tiede B, Massagué J, Kang Y (2007) Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 17(1):3–14
Liu S, Li S, Du Y (2010) Polychlorinated biphenyls (PCBs) enhance metastatic properties of breast cancer cells by activating Rho-associated kinase (ROCK). PLoS ONE 5:e11272
Morgan M, Deoraj A, Felty Q, Roy D (2016) Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol Cell Endocrinol 457:89–102
Muscat JE, Britton JA, Djordjevic MV et al (2003) Adipose concentrations of organochlorine compounds and breast cancer recurrence in Long Island, New York. Cancer Epidemiol Biomark Prev 12:1474–1478
Naik RP, Jin D, Chuang E, Gold EG, Tousimis EA, Moore AL, Christos PJ, de Dalmas T, Donovan D, Rafii S, Vahdat LT (2008) Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat 107(1):133–138. https://doi.org/10.1007/s10549-007-9519-6 (Epub 2007 Feb 15, PMID: 18043899)
Nair R, Teo WS, Mittal V, Swarbrick A (2014) ID proteins regulate diverse aspects of cancer progression and provide novel therapeutic opportunities. Mol Ther 22(8):1407–1415. https://doi.org/10.1038/mt.2014.83
Nelson LR, Bulun SE (2001) Estrogen production and action. J Am Acad Dermatol 45(3 Suppl):S116–S124
Nishi M, Sakai Y, Akutsu H, Nagashima Y, Quinn G, Masui S, Kimura H, Perrem K, Umezawa A, Yamamoto N et al (2014) Induction of cells with cancer stem cell properties from nontumorigenic human mammary epithelial cells by defined reprogramming factors. Oncogene 33:643–652. https://doi.org/10.1038/onc.2012.614
Perciados M, Yoo C, Roy D (2016) Estrogenic endocrine disrupting chemicals influencing NRF1 regulated gene networks in the development of complex human brain diseases. Int J Mol Sci 17(12):2086
Ramos J, Das J, Felty Q, Yoo C, Poppiti R, Murrell D, Foster PJ, Roy D (2018) NRF1 motif sequence-enriched genes involved in ER/PR -ve HER2 +ve breast cancer signaling pathways. Breast Cancer Res Treat 172(2):469–485. https://doi.org/10.1007/s10549-018-4905-9 (Epub 2018 Aug 20 PMID: 30128822)
Roy D, Morgan M, Yoo C et al (2015) Integrated bioinformatics, environmental epidemiologic and genomic approaches to identify environmental and molecular links between endometriosis and breast cancer. Int J Mol Sci 16(10):25285–25322
Sartorius CA, Hanna CT, Gril B et al (2016) Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism. Oncogene 35:2881–2892
Urano Y, Fukushima T, Kitamura S, Mori H, Baba K, Aizawa S. Statistical studies on metastasis of breast cancer and cancer metastasis to the breast. Gan No Rinsho. 1986;Suppl:205–23.
Acknowledgements
This research is supported in part by the NIH R15 Award (1R15HL145652-01).
Funding
This study was funded in part by the NIH R15 Award (1R15HL145652-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, J.K., Deoraj, A., Roy, D. et al. Brain infiltration of breast cancer stem cells is facilitated by paracrine signaling by inhibitor of differentiation 3 to nuclear respiratory factor 1. J Cancer Res Clin Oncol 148, 2881–2891 (2022). https://doi.org/10.1007/s00432-022-04026-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04026-w