Abstract
Introduction
hTERT (human telomerase reverse transcriptase) is a catalytic subunit of the enzyme telomerase and has a role in cell proliferation, cellular senescence, and human aging.
Materials and methods
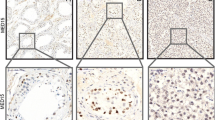
The purpose of this study was to evaluate the expression and significance of hTERT protein expression as a prognostic marker in different histological subtypes of testicular germ cell tumors (TGCTs), including 46 embryonal carcinomas, 46 yolk sac tumors, 38 teratomas, 84 seminomas as well as two main subtypes of seminomas and non-seminomas using tissue microarray (TMA) technique.
Results
The results showed that there is a statistically significance difference between the expression of hTERT and various histological subtypes of TGCTs (P < 0.001). In embryonal carcinoma, low level expression of hTERT protein was significantly associated with advanced pT stage (P = 0.023) as well as tunica vaginalis invasion (P = 0.043). Moreover, low level expression of hTERT protein was found to be a significant predictor of worse DSS (log rank: P = 0.011) and PFS (log rank: P = 0.011) in the univariate analysis. Additionally, significant differences were observed (P =0.021, P =0.018) with 5-year survival rates for DSS and PFS of 66% and 70% for moderate as compared to 97% and 97% for high hTERT protein expression, respectively.
Conclusion
We showed that hTERT protein expression was associated with more aggressive tumor behavior in embryonal carcinoma patients. Also, hTERT may be a novel worse prognostic indicator of DSS or PFS, if the patients are followed up for more time periods.




Similar content being viewed by others
Data availability
The analyzed data during the current study are available from the corresponding author on reasonable request.
References
Albers P et al (2018) Testicular cancer EAU Guidelines. Edn. presented at the EAU Annual Congress Copenhagen. ISBN 978-94-92671-01-1
Bahrami A, Ro JY, Ayala AG (2007) An overview of testicular germ cell tumors. Arch Pathol Lab Med 131:1267–1280
Blackburn EH (2005) Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579:859–862
Campbell L et al (2006) hTERT, the catalytic component of telomerase, is downregulated in the haematopoietic stem cells of patients with chronic myeloid leukaemia. Leukemia 20:671
Chen H-H et al (2007) Expression of human telomerase reverse transcriptase (hTERT) protein is significantly associated with the progression, recurrence and prognosis of oral squamous cell carcinoma in Taiwan. Oral Oncol 43:122–129
Cifuentes-Rojas C, Shippen DE (2012) Telomerase regulation. Mutat Res Fundam Mol Mech Mutagen 730:20–27
Cong Y, Shay JW (2008) Actions of human telomerase beyond telomeres. Cell Res 18:725
Dahse R, Fiedler W, Ernst G (1997) Telomeres and telomerase: biological and clinical importance. Clin Chem 43:708–714
Farmanfarma KK, Mahdavifar N, Mohammadian-Hafshejani A, Salehiniya H (2018) Testicular cancer in the world: an epidemiological review. World Cancer Res J 5(4):e1180
Fung C, Fossa SD, Williams A, Travis LB (2015) Long-term morbidity of testicular cancer treatment. Urol Clin 42:393–408
Hannen R, Bartsch JW (2018) Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett 592:2023–2031
Hiyama E, Hiyama K (2007) Telomere and telomerase in stem cells. Br J Cancer 96:1020
Ibrahim DY, Sun H (2019) Somatic malignant transformation of a testicular teratoma: a case report and an unusual presentation case reports in pathology. Article ID 5273607, 5 pages
Jourdan F et al (2003) Tissue microarray technology: validation in colorectal carcinoma and analysis of p53, hMLH1, and hMSH2 immunohistochemical expression. Virchows Arch 443:115–121
Khattar E et al (2016) Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J Clin Investig 126:4045–4060
Langer R et al (2006) Prognostic significance of expression patterns of c-erbB-2, p53, p16INK4A, p27KIP1, cyclin D1 and epidermal growth factor receptor in oesophageal adenocarcinoma: a tissue microarray study. J Clin Pathol 59:631–634
Leão R, Ahmad AE, Hamilton RJ (2019) Testicular cancer biomarkers: a role for precision medicine in testicular cancer. Clin Genitour Cancer 17:e176–e183
Lu C et al (2004) Prognostic factors in resected stage I non–small-cell lung cancer: a multivariate analysis of six molecular markers. J Clin Oncol 22:4575–4583
Magers MJ, Idrees MT (2018) Updates in staging and reporting of testicular cancer. Surg Pathol Clin 11:813–824
Marchetti A et al (2002) Prediction of survival in stage I lung carcinoma patients by telomerase function evaluation. Lab Investig 82:729
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs—part a: renal, penile, and testicular tumours. Eur Urol 70:93–105
Nguyen LV, Vanner R, Dirks P, Eaves CJ (2012) Cancer stem cells: an evolving concept. Nat Rev Cancer 12:133–143
Nowak R, Sikora K, Piętas A, Skoneczna I, Chrapusta SJ (2000) Germ cell-like telomeric length homeostasis in nonseminomatous testicular germ cell tumors. Oncogene 19:4075–4078
Oldenburg J (2015) Hypogonadism and fertility issues following primary treatment for testicular cancer. In: Urologic oncology: seminars and original investigations 33:407–412
Papaioannou N, Psalla D, Zavlaris M, Loukopoulos P, Tziris N, Vlemmas I (2009) Immunohistochemical expression of dogTERT in canine testicular tumours in relation to PCNA, ki67 and p53 expression. Vet Res Commun 33:905–919
Rajpert-De Meyts E, Skakkebaek NE, Toppari J (2018) Testicular cancer pathogenesis, diagnosis and endocrine aspects. In: Endotext [Internet]. MDText. com, Inc.,
Richie JP (2006) Second cancers among 40,576 testicular cancer patients: focus on long-term survivors: Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD Jr, Dores GM, Gilbert ES, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD. In: Urologic Oncology: Seminars and Original Investigations, 24:171–172
Saeednejad Zanjani L et al (2019a) Spheroid-derived cells from renal adenocarcinoma have low telomerase activity and high stem-like and invasive characteristics. Front Oncol 9:1302
Saeednejad Zanjani L et al (2019b) Human telomerase reverse transcriptase protein expression predicts tumour aggressiveness and survival in patients with clear cell renal cell carcinoma. Pathology 51:21–31
Sanfrancesco JM, Trevino KE, Xu H, Ulbright TM, Idrees MT (2018) The significance of spermatic cord involvement by testicular germ cell tumors. Am J Surg Pathol 42:306–311
Schrader M et al (2002) The differentiation status of primary gonadal germ cell tumors correlates inversely with telomerase activity and the expression level of the gene encoding the catalytic subunit of telomerase. BMC Cancer 2:32
Shay JW, Wright WE (2010) Telomeres and telomerase in normal and cancer stem cells. FEBS Lett 584:3819–3825
Shervington A, Lu C, Patel R, Shervington L (2009) Telomerase downregulation in cancer brain stem cell. Mol Cell Biochem 331:153
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020 CA: a cancer. J Clin 70:7–30
Stephenson A et al (2019) Diagnosis and treatment of early stage testicular cancer: AUA Guideline. J Urol. https://doi.org/10.1097/JU.0000000000000318
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW (1996) Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18:173–179
Yilmaz A, Cheng T, Zhang J, Trpkov K (2013) Testicular hilum and vascular invasion predict advanced clinical stage in nonseminomatous germ cell tumors. Mod Pathol 26:579–586
Zachos I et al (2009) Predictive value of telomerase reverse transcriptase expression in patients with high risk superficial bladder cancer treated with adjuvant BCG immunotherapy. J Cancer Res Clin Oncol 135:1169–1175
Znaor A et al (2019) Testicular cancer incidence predictions in Europe 2010–2035: a rising burden despite population ageing. Int J Cancer 147:820–828
叶哲伟, 陈晓春, 杨述华, 杨秀萍, 曾汉青, 谷龙杰, 鲁功成 (2004) Expression and effects of human telomerase RNA in testicular tumor 中华医学杂志: 英文版 117:941–943
Funding
This work was supported by the grant from Iran University of Medical Sciences (number: 1397-2-28-12348).
Author information
Authors and Affiliations
Contributions
ZM designed and supervised the work; MS gathered the paraffin-embedded tissues, collected the patient data, examined hematoxylin and eosin slides, and marked the most representative areas in different parts of the tumor for preparing the TMA blocks; LS performed the immunohistochemistry examinations, prepared the information of patient survival outcomes, analyzed and interpreted the data as well as wrote the manuscript; MAa, and MAb scored TMA slides after immunohistochemical staining; MR was a consultant on this project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Human Research Ethics Committee of Iran University of Medical Sciences in Iran (Ref no: IR.IUMS.REC1397.443). All the procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants, parents or legally authorized representatives of participants under legal age years old at the time of sample collection with routine consent forms.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
1: Isotype controls in immunohistochemistry staining for confirming nonspecific binding of primary antibody. In embryonal carcinomas (A), yolk sac tumors (B), teratomas (C), seminomas (D), adjacent normal tissue (E), and in benign tumor tissue (F)
Supplementary Fig.
2: Kaplan–Meier curves for disease-specific survival (DSS) and progression-free survival (PFS) based on hTERT protein expression level in non-seminomatous GCT. Kaplan–Meier survival curves showed that low levels of hTERT expression are not significantly related to (A) DSS and (B) PFS (P = 0.161, P = 0.148), respectively in non-seminomatous GCT samples
Rights and permissions
About this article
Cite this article
Shahin, M., Saeednejad Zanjani, L., Abolhasani, M. et al. Low level expression of human telomerase reverse transcriptase predicts cancer-related death and progression in embryonal carcinoma. J Cancer Res Clin Oncol 146, 2753–2775 (2020). https://doi.org/10.1007/s00432-020-03319-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03319-2