Abstract
Purpose
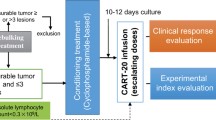
Burkitt lymphoma (BL) is one of the most frequent subtypes of non-Hodgkin lymphoma (NHL) in children. Currently, short, intensive chemotherapy is used internationally and has greatly improved survival in children with BL. However, 5–10% of patients suffer recurrence after intensive chemotherapy, and the prognosis of these patients remains poor. The overall survival rate is only approximately 10%. Innovative therapies are needed to attain a higher rate of remission, such as immunotherapy for relapsed refractory (r/r) BL patients.
Methods
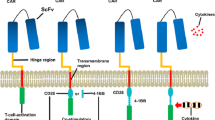
An 8-year-old boy with BL was studied. He suffered a relapse after treatment with standard chemotherapy. Then, we treated this patient using autologous chimeric antigen receptor T-cell (CAR-T) therapies, sequentially targeting antigens CD19, CD22, and CD20. A review of the current literature on CAR-T treatment for lymphoma is presented.
Results
The patient had no discernible response to anti-CD19 CAR-T treatment and exhibited progressive disease (PD). Following CD-22-directed CAR-T treatment, the patient underwent a partial remission (PR), but unfortunately a relapse rapidly occurred. Finally, after administering the anti-CD20 CAR-T cell therapy, the child went into complete remission (CR). The young boy has currently achieved 16-month event-free survival (EFS) so far. During administration of the CD19 and CD20 CAR-T cells, the patient appeared to experience mild (Grade I) cytokine release syndrome (CRS). However, during the CD22 CAR-T therapy, he appeared to experience grade III CRS.
Conclusion
Autologous anti-CD19, anti-CD22, and anti-CD20 CAR-T cell therapies targeting multiple tumor antigens could be an innovative and sound treatment for children with r/r BL, provided that they are closely monitored during treatment.



Similar content being viewed by others
Data availability
Available upon request.
Change history
25 May 2020
In the original article published, the first author���s affiliation is incorrect.
Abbreviations
- BL:
-
Burkitt lymphoma
- CAR-T cell:
-
Chimeric antigen receptor T cell
- CRES:
-
CAR-T cell-related encephalopathy syndrome
- PD:
-
Progressive disease
- PR:
-
Partial remission
- CR:
-
Complete remission
- CRP:
-
C-reactive protein
- CRS:
-
Cytokine release syndrome
- EFS:
-
Event-free survival
- NHL:
-
Non-Hodgkin lymphoma
- PET:
-
Positron emission tomography
- r/r:
-
Relapsed refractory
References
Brentjens RJ, Davila ML, Riviere I, Park JH, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu JR, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy refractory acute lymphoblastic leukemia. Sci Transl Med 5:177ra38
Brudno JN, Kochenderfer JN (2016) Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127:3321–3330
Brudno JN, Kochenderfer JN (2018) Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15:31–46
Budde LE, Berger C, Lin Y, Wang JJ, Frayo SE, Brouns SA, Spencer DM, Till BG, Jensen MC, Riddell SR, Press OW (2013) Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 8:e82742
Du J, Zhang Y (2019) Sequential anti-CD19, anti-CD22, and anti-CD20 autologous chimeric antigen receptor T cell (CAR-T) therapies treating a child with relapsed refractory Burkitt lymphoma. In: 34th Annual meeting and pre-conference programs of the society for immunotherapy of cancer. J Immunother Cancer 7:S1
Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, Shaw P, Berg RA, June CH, Porter DL, Frey NV, Grupp SA, Teachey DT (2017) Cytokine release syndromeafter chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med 45:e124–e131
Fry TJ, Shah NN, Orentas RJ (2018) CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24(1):20–28
Gardner R, Wu D, Cherian S, Fang M, Hanafi L, Finney O, Smithers H, Jensen MC, Riddell SR, Maloney DG, Turtle CJ (2016) Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 127:2406–2410
Gill S, Maus MV, Porter DL (2016) Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev 30:157–167
Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ, Barrett DM, Wayne AS, Mackall CL, Orentas RJ (2013) Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121:1165–1174
Johnson LA, June CH (2017) Driving gene-engineered T cell immunotherapy of cancer. Cell Res 27:38–58
Jourdain A, Auperin A, Minard-Colin V, Aladjidi N, Zsiros J, Coze C (2015) Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “lymphomes malins B” protocols. A société française des cancers de l’enfant study. Haematologica 100:810–817
Kehrl JH, Riva A, Wilson GL, Thevenin C (1994) Molecular mechanisms regulating CD19, CD20 and CD22 gene expression. Immunol Today 15:432–436
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188–195
Liu S, Deng B, Yin Z, Pan J, Lin Y, Ling Z, Wu T, Chen D, Chang AH, Gao Z, Song Y, Zhao Y, Tong C (2020) Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J 10(2):15
Minard-Colin V, Brugieres L, Reiter A, Cairo MS, Gross TG, Woessmann W (2015) Non-hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol 33:2963–2974
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, de Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ (2018) Chimeric antigen receptor T-cell therapy: assessment and management of toxicities. Nat Rev Clin Oncol 15:47–62
Norelli M, Camisa B, Barbiera G et al (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR-T cells. Nat Med 24(6):739–748
Pan J, Niu Q, Deng BP et al (2019) CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 33:2854–2866
Park JH, Riviere I, Wang X, Purdon T, Sadelain M, Brentjens RJ (2016) Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J Clin Oncol 34:7003
Park JH, Riviere I, Gonen M, Wang X, Brigitte S, Currran KJ (2018) Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378(5):449–459
Ramos CA, Heslop HE, Brenner MK (2016) CAR-T cell therapy for lymphoma. Ann Rev Med 67:165–183
Raponi S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L, Perbellini O, Pizzolo G, Foa R, Guarini A (2011) Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma 58:1098–1107
Reagan JL, Fast LD, Safran H, Nevola M, Winer ES, Castillo JJ (2013) Cellular immunotherapy for refractory hematological malignancies. J Transl Med 11:150
Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE (2018) Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med 24:1499–1503
Schneider D, Xiong Y, Wu D, Nolle V, Haso W (2017) A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer 5(1):42
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, Martinez NM, Harrington CT, Chung EY, Perazzelli J, Hofmann TJ, Maude SL, Raman P, Barrera A, Gill S, Lacey SF, Melenhorst JJ, Allman D, Jacoby E, Fry T, Mackall C, Barash Y, Lynch KW, Maris JM, Grupp SA, Thomas-Tikhonenko A (2015) Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 5:1282–1295
Srivastava S, Riddell SR (2015) Engineering CAR-T cells: design concepts. Trends Immunol 36:494–502
US Department of Health and Human Services (2010) Common terminology criteria for adverse events. V4.03: 14 June 2010
Xu XJ, Tang YM (2014) Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett 343:172–178
Acknowledgements
We gratefully acknowledge Bo Hu, Wenqun Zhang, and other members of Children’s Lymphoma Ward, Beijing Boren Hospital for their invaluable assistance throughout the preparation of original manuscript. Many thanks to Ling Jin and Jing Yang (Department of Hematology Oncology, National Center for Children’s Health, Beijing Children’s Hospital) providing technical assistance and giving us advice of great value.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JD drafted the manuscript. YZ revised and edited the manuscript multiple times. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Informed consent
Written informed consent was obtained from the patient’s guardian for the analysis of the samples and the tissues. Consent for publication was obtained from the patient’s guardian.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, J., Zhang, Y. Sequential anti-CD19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: a case report and literature review. J Cancer Res Clin Oncol 146, 1575–1582 (2020). https://doi.org/10.1007/s00432-020-03198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03198-7