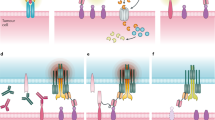
Abstract
Purpose
Globally, cancer is a critical illness which seriously threatens human health. T-cell-based cancer immunotherapy for some patients has demonstrated impressive achievements including chimeric antigen receptor T cells, immune checkpoint inhibitors and T cell-redirecting bispecific antibodies (TRBAs). TRBAs recruit T cells to lyse cancer cells bypassing the antigen presentation through the major histocompatibility complex pathways. In this review we summarized the TRBAs formats, biophysical characteristics, the preclinical and clinical trial results, as well as the challenges faced by TRBAs in tumour therapy.
Methods
Herein the relevant literature and clinical trials from the PubMed and ClinicalTrials.gov database.
Results
The advances in protein engineering technology have generated diverse TRBAs format which can be classified into two categories: IgG-like TRBAs and non-IgG-like TRBAs. Multiple applications of TRBAs showed encouraging curative effect and entered clinical trials for lymphoid malignancy and solid tumour.
Conclusions
TRBA is a powerful tool for the cancer treatment and the clinical studies showed potent anti-tumour efficacy in hematologic malignancies. Although the clinical outcomes of TRBAs in solid tumours are less satisfied than hematologic malignancies, many preclinical antibodies and combination therapies are being evaluated


Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myelocytic leukemia
- APCs:
-
Antigen-presenting cells
- ATC:
-
Activated T cells
- BiTE:
-
Bispecific T-cell engagers
- BsAb:
-
Bispecific antibody
- CAR-T:
-
Chimeric antigen receptor T cells
- CDC:
-
Complement-dependent cytotoxicity
- CEA:
-
Carcinoembryonic antigen
- CLL:
-
Chronic lymphocytic leukemia
- CR:
-
Complete response
- CRh:
-
Complete response with partial hematopoietic recovery
- DART:
-
Dual-affinity re-targeting
- DLBCL:
-
Diffuse large B-cell lymphoma
- EpCAM:
-
Epithelial cell adhesion molecule
- FAE:
-
Fab-arm exchange
- Fab:
-
Fragment of antigen binding
- FcγR:
-
Fc-gamma receptors
- Fv:
-
Variable fragments
- HER:
-
Human epidermal growth factor receptor
- ImmTAC:
-
Immune-mobilising monoclonal T cell receptors against cancer
- KiH:
-
Knobs-into-holes
- mCR:
-
Macroscopic complete remission
- MDS:
-
Myelodysplastic syndrome
- MHC:
-
Major histocompatibility complex
- MM:
-
Multiple myeloma
- MRD:
-
Minimal residual disease
- MTD:
-
Maximum tolerated dose
- NETs:
-
Neuroendocrine tumours
- NHL:
-
Non-Hodgkin lymphoma
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- Ph−:
-
Philadelphia chromosome negative
- Ph+:
-
Philadelphia chromosome positive
- PSMA:
-
Prostate-specific membrane antigen
- ScFv:
-
Single chain antibody variable fragments
- SSTR2:
-
Somatostatin receptor 2
- SOC:
-
Standard of care chemotherapy
- TCR:
-
T cell receptor
- TRBAs:
-
T cell-redirecting bispecific antibodies
References
Aigner M et al (2013) T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia 27:1107–1115. https://doi.org/10.1038/leu.2012.341
Al-Hussaini M et al (2016) Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity. retargeting platform. Blood 127:122–131. https://doi.org/10.1182/blood-2014-05-575704
Bacac M et al (2016) A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the Treatment of solid tumors. Clin Cancer Res 22:3286–3297. https://doi.org/10.1158/1078-0432.CCR-15-1696
Banchereau J, Palucka K (2018) Immunotherapy: cancer vaccines on the move. Nat Rev Clin Oncol 15:9–10. https://doi.org/10.1038/nrclinonc.2017.149
Bannerji R et al (2016) Phase 1 study of REGN1979, an anti-CD20 × anti-CD3 bispecific monoclonal antibody, in patients with CD20 + B-cell malignancies previously treated with CD20-directed antibody therapy. Blood 128:621–621
Barlev A, Lin VW, Katz A, Hu K, Cong Z, Barber B (2017) Estimating long-term survival of adults with philadelphia chromosome-negative relapsed/refractory B-precursor acute lymphoblastic leukemia treated with blinatumomab using historical data. Adv Ther 34:148–155. https://doi.org/10.1007/s12325-016-0447-x
Beatty GL, Gladney WL (2015) Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res 21:687–692. https://doi.org/10.1158/1078-0432.CCR-14-1860
Blankenship JW et al (2016) Abstract 4995: anti-ROR1 x anti-CD3 ADAPTIR™ molecule, ES425, redirects T-cell cytotoxicity and inhibits tumor growth in preclinical models of triple-negative breast cancer. Can Res 76:4995–4995. https://doi.org/10.1158/1538-7445.am2016-4995
Bossi G, Buisson S, Oates J, Jakobsen BK, Hassan NJ (2014) ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol Immunother CII 63:437–448. https://doi.org/10.1007/s00262-014-1525-z
Buhmann R, Michael S, Juergen H, Horst L, Peschel C, Kolb HJ (2013) Immunotherapy with FBTA05 (Bi20), a trifunctional bispecific anti-CD3 × anti-CD20 antibody and donor lymphocyte infusion (DLI) in relapsed or refractory B-cell lymphoma after allogeneic stem cell transplantation: study protocol of an investigator-driven, open-label, non-randomized, uncontrolled, dose-escalating Phase I/II-trial. J Transl Med. https://doi.org/10.1186/1479-5876-11-160
Byun DJ, Wolchok JD, Rosenberg LM, Girotra M (2017) Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 13:195. https://doi.org/10.1038/nrendo.2016.205
Cameron F, Whiteside G, Perry C (2011) Ipilimumab: first global approval. Drugs 71:1093–1104. https://doi.org/10.2165/11594010-000000000-00000
Chelius D et al (2010) Structural and functional characterization of the trifunctional antibody catumaxomab. mAbs 2:309–319 https://doi.org/10.4161/mabs.2.3.11791
Cheng P et al (2017) Immunodepletion of MDSC by AMV564, a novel tetravalent bispecific CD33/CD3 T cell engager restores immune homeostasis in MDS in vitro. Blood 130:51–51
Chichili GR et al (2015) A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaa5693
Chu SY et al (2014a) Immunotherapy with long-lived anti-CD20 × anti-CD3 bispecific antibodies stimulates potent T cell-mediated killing of human B cell lines and of circulating and lymphoid B cells in monkeys: a potential therapy for B cell lymphomas and Leukemias. Blood 124:3111–3111
Chu SY et al (2014b) Immunotherapy with long-lived anti-CD123 × anti-CD3 bispecific antibodies stimulates potent T cell-mediated killing of human AML cell lines and of CD123 + cells in monkeys: a potential therapy for acute myelogenous Leukemia. Blood 124:2316–2316
Comeau MR et al (2018) Abstract 1786: APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity, induces potent T-cell activation, proliferation and cytotoxicity with limited cytokine release. Can Res 78:1786–1786. https://doi.org/10.1158/1538-7445.am2018-1786
de Zafra C et al (2017) Preclinical characterization of AMG 424, a novel humanized T cell-recruiting bispecific anti-CD3/CD38. Antib Blood 130:500–500
Ellwanger K et al (2017) Highly specific and effective targeting of EGFRvIII-positive tumors with TandAb. Antibod Front Oncol 7:100. https://doi.org/10.3389/fonc.2017.00100
Esteban RE, Christianne B, Alvaro A, Demichelis-Gomez R (2018) Prognostic effect of CD20 expression in Adult B-cell acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 18:361–367. https://doi.org/10.1016/j.clml.2018.02.013
Fan G, Wang Z, Hao M, Li J (2015) Bispecific antibodies and their applications. J Hematol Oncol. https://doi.org/10.1186/s13045-015-0227-0
Ferl GZ, Reyes A, Sun LL, Cheu M, Oldendorp A, Ramanujan S, Stefanich EG (2018) A preclinical population pharmacokinetic model for anti-CD20/CD3 T-cell-dependent bispecific antibodies. Clin Transl Sci 11:296–304 https://doi.org/10.1111/cts.12535
Fiedler WM et al (2010) Phase I safety and pharmacology study of the EpCAM/CD3-bispecific BiTE antibody MT110 in patients with metastatic colorectal, gastric, or lung cancer. J Clin Oncol 28:2573–2573. https://doi.org/10.1200/jco.2010.28.15_suppl.2573
Fiedler WM et al (2012) A phase I study of EpCAM/CD3-bispecific antibody (MT110) in patients with advanced solid tumors. J Clin Oncol 30:2504–2504. https://doi.org/10.1200/jco.2012.30.15_suppl.2504
Fisher TS et al (2018) A CD3-bispecific molecule targeting P-cadherin demonstrates T cell-mediated regression of established solid tumors in mice. Cancer Immunol Immunother CII 67:247–259. https://doi.org/10.1007/s00262-017-2081-0
Fraietta JA et al (2018) Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 24:563–571. https://doi.org/10.1038/s41591-018-0010-1
Friedrich M et al (2012) Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther 11:2664–2673. https://doi.org/10.1158/1535-7163.mct-12-0042
Gaudet F et al (2016) Development of a CD123 × CD3 bispecific antibody (JNJ-63709178) for the treatment of acute myeloid leukemia (AML). Blood 128:2824–2824
Ghosh A, Heston WDW (2004) Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 91:528–539. https://doi.org/10.1002/jcb.10661
Godwin CD, Bates OM, Laszlo GS, Gottschalk R, Comeau MR, Hoyos GH, Walter RB (2017) Bispecific anti-CD123 × anti-CD3 Adaptir™ molecules APVO436 and APVO437 have broad activity against primary human AML cells in vitro. Blood 130:2639–2639
Goebeler ME et al (2016) Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase I study. J Clin Oncol 34:1104–1111. https://doi.org/10.1200/JCO.2014.59.1586
Gokbuget N, Dombret H (2018) Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 131:1522–1531 https://doi.org/10.1182/blood-2017-08-798322
Haense N et al (2016) A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer 16:420. https://doi.org/10.1186/s12885-016-2449-0
Hallet J, Law CHL, Cukier M, Saskin R, Liu N, Singh S (2015) Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation and outcomes. Cancer 121:589–597. https://doi.org/10.1002/cncr.29099
Han TH, Guenot J, Denney WS, Feldman EJ (2018) The therapeutic potential of AMV564, a novel bispecific bivalent (2 × 2) T-cell engager, for the treatment of CD33-expressing hematologic malignancies. Can Res 78:5548–5548. https://doi.org/10.1158/1538-7445.am2018-5548
Hassan NJ et al (2014) Abstract 3525: IMCgp100: a novel bi-specific biologic for the treatment of malignant melanoma. Can Res 72:3525–3525. https://doi.org/10.1158/1538-7445.am2012-3525
Hauswirth AW et al (2007) Expression of the target receptor CD33 in CD34+/CD38−/CD123 + AML stem cells. Eur J Clin Investig 37:73–82. https://doi.org/10.1111/j.1365-2362.2007.01746.x
Hernandez-Hoyos G et al (2016) MOR209/ES414, a novel bispecific antibody targeting PSMA for the treatment of metastatic castration-resistant prostate cancer. Mol Cancer Ther 15:2155–2165. https://doi.org/10.1158/1535-7163.MCT-15-0242
Herrmann I et al (2010) Highly efficient elimination of colorectal tumor-initiating cells by an EpCAM/CD3-bispecific antibody engaging human T cells. PLoS One 5:e13474 https://doi.org/10.1371/journal.pone.0013474
Hipp S et al (2017) A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 31:1743–1751. https://doi.org/10.1038/leu.2016.388
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S (2014) Drug resistance in cancer: an overview. Cancers 6:1769
Ishiguro T et al (2017) An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aal4291
Johnson S et al (2010) Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 399:436–449. https://doi.org/10.1016/j.jmb.2010.04.001
Kantarjian H et al (2017) Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376:836–847. https://doi.org/10.1056/NEJMoa1609783
Kebenko M et al (2018) A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. https://doi.org/10.1080/2162402x.2018.1450710
Kiewe P et al (2006) Phase I trial of the trifunctional anti-HER2 × anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res 12:3085–3091. https://doi.org/10.1158/1078-0432.CCR-05-2436
Klausen U, Jorgensen NGD, Grauslund JH, Holmstrom MO, Andersen MH (2018) Cancer immune therapy for lymphoid malignancies: recent advances. Semin Immunopathol. https://doi.org/10.1007/s00281-018-0696-7
Klinger M et al (2012) Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 119:6226–6233. https://doi.org/10.1182/blood-2012-01-400515
Knodler M et al (2018) Randomised phase II trial to investigate catumaxomab (anti-EpCAM × anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer. Br J Cancer. https://doi.org/10.1038/s41416-018-0150-6
Labrijn AF et al (2011) Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3–CH3 interaction strength. J Immunol 187:3238–3246. https://doi.org/10.4049/jimmunol.1003336
Labrijn AF et al (2013) Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci USA 110:5145–5150. https://doi.org/10.1073/pnas.1220145110
Lee S-H et al. (2017a) Abstract 3633: Anti-SSTR2 × anti-CD3 bispecific antibody induces potent killing of human tumor cells in vitro and in mice, and stimulates target-dependent T cell activation in monkeys: a potential immunotherapy for neuroendocrine tumors. Cancer Res 77:3633–3633 https://doi.org/10.1158/1538-7445.am2017-3633
Lee S-H et al (2017b) Abstract 3633: Anti-SSTR2 × anti-CD3 bispecific antibody induces potent killing of human tumor cellsin vitroand in mice, and stimulates target-dependent T cell activation in monkeys: a potential immunotherapy for neuroendocrine tumors. Can Res 77:3633–3633. https://doi.org/10.1158/1538-7445.am2017-3633
Lehmann S et al (2016) In vivo fluorescence imaging of the activity of CEA TCB, a novel T-cell bispecific antibody, reveals highly specific tumor targeting and fast induction of T-cell-mediated tumor killing. Clin Cancer Res 22:4417–4427. https://doi.org/10.1158/1078-0432.CCR-15-2622
Liu L et al (2017) MGD011, A CD19 × CD3 dual-affinity retargeting bi-specific molecule incorporating extended circulating half-life for the treatment of B-cell malignancies. Clin Cancer Res 23:1506–1518. https://doi.org/10.1158/1078-0432.CCR-16-0666
Loffler A et al (2000) A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 95:2098–2103
Lum LG et al (2015) Targeted T-cell therapy in stage IV breast cancer: a phase I clinical trial. Clin Cancer Res 21:2305–2314. https://doi.org/10.1158/1078-0432.CCR-14-2280
Martinelli G et al (2017) Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome—positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol 35:1795–1802. https://doi.org/10.1200/jco.2016.69.3531
McAleese F, Eser M (2012) RECRUIT-TandAbs: harnessing the immune system to kill cancer cells. Fut Oncol 8:687–695. https://doi.org/10.2217/fon.12.54
Melero I, Rouzaut A, Motz GT, Coukos G (2014) T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov 4:522–526. https://doi.org/10.1158/2159-8290.CD-13-0985
Middleton MR et al (2016) Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific T cell redirector with solid tumour activity: results from the FIH study in melanoma. J Clin Oncol 34:3016–3016. https://doi.org/10.1200/JCO.2016.34.15_suppl.3016
Moore PA et al (2011) Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 117:4542–4551. https://doi.org/10.1182/blood-2010-09-306449
Moore PA et al (2018) Development of MGD007, a gpA33 × CD3 bispecific DART(R) protein for T-cell immunotherapy of metastatic colorectal cancer. Mol Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-17-1086
Moretti P et al (2013) BEAT® the bispecific challenge: a novel and efficient platform for the expression of bispecific IgGs. BMC Proc 7:O9. https://doi.org/10.1186/1753-6561-7-s6-o9
Munoz L et al (2001) Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica 86:1261–1269
Oates J, Hassan NJ, Jakobsen BK (2015) ImmTACs for targeted cancer therapy: why, what, how, and which. Mol Immunol 67:67–74 https://doi.org/10.1016/j.molimm.2015.01.024
Oberst MD et al (2014) CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. mAbs 6:1571–1584 https://doi.org/10.4161/19420862.2014.975660
Ogita Y et al (2018) A phase 1 dose escalation (DE) and cohort expansion (CE) study of ERY974, an anti-Glypican 3 (GPC3)/CD3 bispecific antibody, in patients with advanced solid tumors. J Clin Oncol 36:TPS2599–TPS2599. https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS2599
Oldhafer KJ, Donati M, Jenner RM, Stang A, Stavrou GA (2014) ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg 38:1504–1509. https://doi.org/10.1007/s00268-013-2401-2
Pellerin L, Chen P, Gregori S, Hernandez-Hoyos G, Bacchetta R, Roncarolo MG (2018) APVO210: a bispecific anti-CD86-IL-10 fusion protein (ADAPTIR™) to induce antigen-specific T regulatory type 1 cells. Front Immunol. https://doi.org/10.3389/fimmu.2018.00881
Picarda E, Ohaegbulam KC, Zang X (2016) Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res 22:3425–3431. https://doi.org/10.1158/1078-0432.CCR-15-2428
Pillarisetti K et al (2016) Development of a New BCMAxCD3 Duobody® antibody for multiple myeloma. Blood 128:2116–2116
Pishvaian M et al (2016) Phase 1 dose escalation study of MEDI-565, a bispecific T-cell engager that targets human carcinoembryonic antigen, in patients with advanced gastrointestinal adenocarcinomas. Clin Colorectal Cancer 15:345–351. https://doi.org/10.1016/j.clcc.2016.07.009
Poole RM (2014) Pembrolizumab: first global approval. Drugs 74:1973–1981. https://doi.org/10.1007/s40265-014-0314-5
Prasad V (2018) Immunotherapy: tisagenlecleucel—the first approved CAR-T-cell therapy: implications for payers and policy makers. Nat Rev Clin Oncol 15:11–12. https://doi.org/10.1038/nrclinonc.2017.156
Przepiorka D et al (2015) FDA approval: blinatumomab. Clin Cancer Res 21:4035–4039. https://doi.org/10.1158/1078-0432.CCR-15-0612
Pulte ED et al (2018) FDA supplemental approval: blinatumomab for treatment of relapsed and refractory precursor B-cell acute lymphoblastic leukemia. Oncologist 23:1366–1371. https://doi.org/10.1634/theoncologist.2018-0179
Reusch U et al (2015) A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. mAbs 7:584–604 https://doi.org/10.1080/19420862.2015.1029216
Ridgway JB, Presta LG, Carter P (1997) Knobs-into-holes’ engineering of antibody CH3 domains for heavy. Protein Eng 9:617–621
Ruella M et al (2016) Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Investig 126:3814–3826. https://doi.org/10.1172/JCI87366
Ryan Richter J et al (2018) Phase 1, multicenter, open-label study of single-agent bispecific antibody T-cell engager GBR 1342 in relapsed/refractory multiple myeloma. J Clin Oncol. https://doi.org/10.1200/JCO.2018.36.5_suppl.TPS81
Sato T et al (2017) Intra-patient escalation dosing strategy with IMCgp100 results in mitigation of T-cell based toxicity and preliminary efficacy in advanced uveal melanoma. J Clin Oncol 35:9531–9531. https://doi.org/10.1200/JCO.2017.35.15_suppl.9531
Schaefer W et al (2011) Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci USA 108:11187–11192. https://doi.org/10.1073/pnas.1019002108
Seimetz D, Lindhofer H, Bokemeyer C (2010) Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 36:458–467. https://doi.org/10.1016/j.ctrv.2010.03.001
Sen M, Wankowski DM, Garlie NK, Siebenlist RE, Van Epps D, LeFever AV, Lum LG (2001) Use of anti-CD3 x anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu + tumors. J Hematother Stem Cell 10:247–260. https://doi.org/10.1089/15258160151134944
Shankar S et al (2018) A phase 1, open label, dose escalation study of MGD009, a humanized B7-H3 × CD3 DART protein, in combination with MGA012, an anti-PD-1 antibody, in patients with relapsed or refractory B7-H3-expressing tumors. J Clin Oncol 36:TPS2601–TPS2601. https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS2601
Shatz W et al (2013) Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity. mAbs 5:872–881 https://doi.org/10.4161/mabs.26307
Shepard HM, Phillips GL, C DT, Feldmann M (2017) Developments in therapy with monoclonal antibodies and related proteins. Clin Med 17:220–232. https://doi.org/10.7861/clinmedicine.17-3-220
Smith EJ et al (2015) A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep 5:17943 https://doi.org/10.1038/srep17943
Spiess C, Zhai Q, Carter PJ (2015) Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 67:95–106. https://doi.org/10.1016/j.molimm.2015.01.003
Staerz UD, Kanagawa O, Bevan MJ (1985) Hybrid antibodies can target sites for attack by T-cells. Nature 314:628–631. https://doi.org/10.1038/314628a0
Stanglmaier M, Faltin M, Ruf P, Bodenhausen A, Schroder P, Lindhofer H (2008) Bi20 (fBTA05), a novel trifunctional bispecific antibody (anti-CD20 × anti-CD3), mediates efficient killing of B-cell lymphoma cells even with very low CD20 expression levels. Int J Cancer 123:1181–1189. https://doi.org/10.1002/ijc.23626
Sun LL et al (2015) Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 7:287ra270. https://doi.org/10.1126/scitranslmed.aaa4802
Tabernero J et al (2017) Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J Clin Oncol 35:3002–3002. https://doi.org/10.1200/JCO.2017.35.15_suppl.3002
Thakur A, Huang M, Lum LG (2018) Bispecific antibody based therapeutics strengths challenges. Blood Rev 32:339–347. https://doi.org/10.1016/j.blre.2018.02.004
Tolcher AW et al (2016) Phase 1, first-in-human, open label, dose escalation ctudy of MGD009, a humanized B7-H3 × CD3 dual-affinity re-targeting (DART) protein in patients with B7-H3-expressing neoplasms or B7-H3 expressing tumor vasculature. J Clin Oncol 34:TPS3105–TPS3105. https://doi.org/10.1200/JCO.2016.34.15_suppl.TPS3105
Topp MS et al (2014) Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 32:4134–4140. https://doi.org/10.1200/jco.2014.56.3247
Topp MS et al (2015) Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 16:57–66. https://doi.org/10.1016/s1470-2045(14)71170-2
Topp MS et al (2017) Safety and preliminary antitumor activity of the anti-PD-1 monoclonal antibody REGN2810 alone or in combination with REGN1979, an anti-CD20 × anti-CD3 bispecific antibody, in patients with B-lymphoid malignancies. Blood 130:1495–1495
Van Loo PF, Doornbos R, Dolstra H, Shamsili S, Bakker L (2015) Preclinical evaluation of MCLA117, a CLEC12AxCD3 bispecific antibody efficiently targeting a novel leukemic stem cell associated antigen AML. Blood 126:325–325
Varghese B et al (2014) A novel CD20xCD3 bispecific fully human antibody induces potent anti-tumor effects against B cell lymphoma in Mice. Blood 124:4501–4501
Viardot A et al (2016) Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 127:1410–1416. https://doi.org/10.1182/blood-2015-06-651380
von Stackelberg A et al (2016) Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381–4389. https://doi.org/10.1200/jco.2016.67.3301
Vries ED et al (2015) Phase I study of AMG 211/MEDI-565 administered as continuous intravenous infusion for relapsed/refractory gastrointestinal (GI) adenocarcinoma. J Clin Oncol 33:TPS3097–TPS3097. https://doi.org/10.1200/jco.2015.33.15_suppl.tps3097
Wang M, Yin B, Wang HY, Wang RF (2014) Current advances in T-cell-based cancer immunotherapy. Immunotherapy Uk 6:1265–1278. https://doi.org/10.2217/imt.14.86
Watkins MP, Bartlett NL (2018) CD19-targeted immunotherapies for treatment of patients with non-Hodgkin B-cell lymphomas. Expert Opin Investig Drugs 27:601–611 https://doi.org/10.1080/13543784.2018.1492549
Wermke M, Schmidt H, Ochsenreither S, Back J, Salhi Y, Bayever E (2016) 52TiPA phase 1 study of GBR 1302 in subjects with HER2-positive cancers. Ann Oncol. https://doi.org/10.1093/annonc/mdw525.52
Wermke M, Schmidt H, Nolte H, Ochsenreither S, Back J, Salhi Y, Bayever E (2017) A phase I study of the bispecific antibody T-cell engager GBR 1302 in subjects with HER2-positive cancers. J Clin Oncol 35:TPS3091–TPS3091. https://doi.org/10.1200/JCO.2017.35.15_suppl.TPS3091
Wermke M et al (2018) Preliminary biomarker and pharmacodynamic data from a phase I study of single-agent bispecific antibody T-cell engager GBR 1302 in subjects with HER2-positive cancers. J Clin Oncol 36:69–69. https://doi.org/10.1200/JCO.2018.36.5_suppl.69
Yankelevich M, Kondadasula SV, Thakur A, Buck S, Cheung NK, Lum LG (2012) Anti-CD3 × anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer 59:1198–1205. https://doi.org/10.1002/pbc.24237
Yu S et al (2017) Recent advances of bispecific antibodies in solid tumors. J Hematol Oncol 10:155. https://doi.org/10.1186/s13045-017-0522-z
Yuraszeck T, Kasichayanula S, Benjamin JE (2017) Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin Pharmacol Ther 101:634–645. https://doi.org/10.1002/cpt.651
Zitron IM, Thakur A, Norkina O, Barger GR, Lum LG, Mittal S (2013) Targeting and killing of glioblastoma with activated T cells armed with bispecific antibodies. BMC Cancer 13:83. https://doi.org/10.1186/1471-2407-13-83
Acknowledgements
This work was supported by the National Key Scientific Instrument and Equipment Development Project (No. 21827812) and the Foundation and Advanced Research Project of CQ CSTC (cstc2018jscx-mszd0280, cstc2017shms-xdny0033, cstc2013jjB0011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests associated with the manuscript.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, L., Wang, J. T cell-redirecting bispecific antibodies in cancer immunotherapy: recent advances. J Cancer Res Clin Oncol 145, 941–956 (2019). https://doi.org/10.1007/s00432-019-02867-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02867-6