Abstract
Purpose
Activated CDC42-associated kinase-1 (ACK1/TNK2) and epigenetic regulators of the histone deacetylase (HDAC) family regulate the proliferation and survival of leukemic cells. 18 HDACs fall into four classes (I–IV). We tested the impact of clinically relevant histone deacetylase inhibitors (HDACi) on ACK1 and if such drugs combine favorably with the therapeutically used ACK1 inhibitor Dasatinib.
Methods
We applied the broad-range HDACi Panobinostat/LBH589 and the class I HDAC-specific inhibitor Entinostat/MS-275 to various acute and chronic myeloid leukemia cells (AML/CML). We also used the replicative stress inducer Hydroxyurea (HU), a standard drug for leukemic patients, and the apoptosis inducer Staurosporine (STS). To assess cytotoxic effects of HDACi, we measured cell cycle profiles and DNA fragmentation by flow cytometry. Western blot was employed to analyze protein expression and phosphorylation.
Results
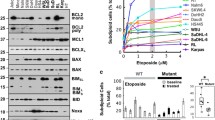
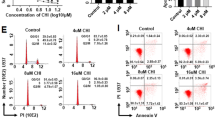
LBH589 and MS-275 induce proteolysis of ACK1 in CML and AML cells. Panobinostat more strongly induces apoptosis than Entinostat, and this correlates with a significantly pronounced loss of ACK1. STS and HU also propel the degradation of ACK1 in leukemic cells. Moreover, the caspase inhibitor z-VAD-FMK reduces ACK1 degradation in the presence of HDACi. Concomitant with the attenuation of ACK1, we noticed decreased phosphorylation of STAT3. Direct inhibition of ACK1 with Dasatinib also suppresses STAT3 phosphorylation. Furthermore, Dasatinib and HDACi combinations are effective against CML cells.
Conclusion
HDACs sustain the ACK1-STAT3 signaling node and leukemic cell growth. Consistent with their different effects on ACK1 stability or auto-phosphorylation, Dasatinib and HDACi combinations produce beneficial antileukemic effects.





Similar content being viewed by others
References
Afifi S, Michael A, Azimi M, Rodriguez M, Lendvai N, Landgren O (2015) Role of histone deacetylase inhibitors in relapsed refractory multiple myeloma: a focus on vorinostat and panobinostat. Pharmacotherapy 35:1173–1188. doi:10.1002/phar.1671
Ahmad M et al (2012) Understanding histone deacetylases in the cancer development and treatment: an epigenetic perspective of cancer chemotherapy. DNA Cell Biol 31(Suppl 1):S62–S71. doi:10.1089/dna.2011.1575
Boschelli F, Arndt K, Gambacorti-Passerini C (2010) Bosutinib: a review of preclinical studies in chronic myelogenous leukaemia. Eur J Cancer 46:1781–1789. doi:10.1016/j.ejca.2010.02.032
Bradner JE et al (2010) Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease. Proc Natl Acad Sci USA 107:12617–12622. doi:10.1073/pnas.1006774107
Buchwald M, Pietschmann K, Müller JP, Böhmer FD, Heinzel T, Krämer OH (2010) Ubiquitin conjugase UBCH8 targets active FMS-like tyrosine kinase 3 for proteasomal degradation. Leukemia 24:1412–1421. doi:10.1038/leu.2010.114
Buchwald M, Pietschmann K, Brand P, Gunther A, Mahajan NP, Heinzel T, Krämer OH (2013) SIAH ubiquitin ligases target the nonreceptor tyrosine kinase ACK1 for ubiquitinylation and proteasomal degradation. Oncogene 32:4913–4920. doi:10.1038/onc.2012.515
Chan D et al (2013) Belinostat and panobinostat (HDACI): in vitro and in vivo studies in thyroid cancer. J Cancer Res Clin Oncol 139:1507–1514. doi:10.1007/s00432-013-1465-6
Cheriyath V, Kuhns MA, Kalaycio ME, Borden EC (2011) Potentiation of apoptosis by histone deacetylase inhibitors and doxorubicin combination: cytoplasmic cathepsin B as a mediator of apoptosis in multiple myeloma. Br J Cancer 104:957–967. doi:10.1038/bjc.2011.42
Corey EJ, Li WD (1999) Total synthesis and biological activity of lactacystin, omuralide and analogs. Chem Pharm Bull 47:1–10
Fujimoto Y et al (2011) A single nucleotide polymorphism in activated Cdc42 associated tyrosine kinase 1 influences the interferon therapy in hepatitis C patients. J Hepatol 54:629–639. doi:10.1016/j.jhep.2010.07.021
Göttlicher M (2004) Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol 83(Suppl 1):S91–S92. doi:10.1007/s00277-004-0850-2
Göttlicher M et al (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20:6969–6978
Graham JS, Kaye SB, Brown R (2009) The promises and pitfalls of epigenetic therapies in solid tumours. Eur J Cancer 45:1129–1136. doi:10.1016/j.ejca.2009.01.003
Hennig D et al (2015) Antagonism between granulocytic maturation and deacetylase inhibitor-induced apoptosis in acute promyelocytic leukaemia cells. Br J Cancer 112:329–337. doi:10.1038/bjc.2014.589
Karaca M, Liu Y, Zhang Z, De Silva D, Parker JS, Earp HS, Whang YE (2015) Mutation of androgen receptor N-terminal phosphorylation site Tyr-267 leads to inhibition of nuclear translocation and DNA binding. PLoS ONE 10:e0126270. doi:10.1371/journal.pone.0126270
Knauer SK, Mahendrarajah N, Roos WP, Krämer OH (2015) The inducible E3 ubiquitin ligases SIAH1 and SIAH2 perform critical roles in breast and prostate cancers. Cytokine Growth Factor Rev 26:405–413. doi:10.1016/j.cytogfr.2015.04.002
Krämer OH, Stauber RH, Bug G, Hartkamp J, Knauer SK (2013) SIAH proteins: critical roles in leukemogenesis. Leukemia 27:792–802. doi:10.1038/leu.2012.284
Licht V et al (2014) Caspase-3 and caspase-6 cleave STAT1 in leukemic cells. Oncotarget 5:2305–2317
Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE (2010) Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene 29:3208–3216. doi:10.1038/onc.2010.103
Ma N, Luo Y, Wang Y, Liao C, Ye WC, Jiang S (2016) Selective histone deacetylase inhibitors with anticancer activity. Curr Top Med Chem 16:415–426
Mace ML, Dahl J, Jabbour EJ (2015) Which tyrosine-kinase inhibitor to use first in chronic phase chronic myelogenous leukemia? Expert Opin Pharmacother 16:999–1007. doi:10.1517/14656566.2015.1031107
Mahajan K, Mahajan NP (2015) ACK1/TNK2 tyrosine kinase: molecular signaling and evolving role in cancers. Oncogene 34:4162–4167. doi:10.1038/onc.2014.350
Mahajan K et al (2012) Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer. J Biol Chem 287:22112–22122. doi:10.1074/jbc.M112.357384
Mahajan K, Lawrence HR, Lawrence NJ, Mahajan NP (2014) ACK1 tyrosine kinase interacts with histone demethylase KDM3A to regulate the mammary tumor oncogene HOXA1. J Biol Chem 289:28179–28191. doi:10.1074/jbc.M114.584425
Maxson JE et al (2013) Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med 368:1781–1790. doi:10.1056/NEJMoa1214514
Maxson JE et al (2016) Identification and characterization of tyrosine kinase nonreceptor 2 mutations in leukemia through integration of kinase inhibitor screening and genomic analysis. Cancer Res 76:127–138. doi:10.1158/0008-5472.CAN-15-0817
Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK (2014) Autophagy and apoptosis: Where do they meet? Apoptosis Int J Program Cell Death 19:555–566. doi:10.1007/s10495-014-0967-2
Müller S, Krämer OH (2010) Inhibitors of HDACs—Effective drugs against cancer? Curr Cancer Drug Targets 10:210–228
Newbold A et al (2013) Molecular and biologic analysis of histone deacetylase inhibitors with diverse specificities. Mol Cancer Ther 12:2709–2721. doi:10.1158/1535-7163.MCT-13-0626
Nonami A et al (2015) Identification of novel therapeutic targets in acute leukemias with NRAS mutations using a pharmacologic approach. Blood 125:3133–3143. doi:10.1182/blood-2014-12-615906
Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, Tsuchya H, Takahashi Y, Masuma R (1977) A new alkaloid AM-2282 OF Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J Antibiot (Tokyo) 30(4):275–282
Rozman-Pungercar J et al (2003) Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ 10:881–888. doi:10.1038/sj.cdd.4401247
Seto E, Yoshida M (2014) Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor Perspect Biol 6:a018713. doi:10.1101/cshperspect.a018713
Shah NP et al (2016) Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. doi:10.1002/ajh.24423
Starkova J et al (2007) The identification of (ETV6)/RUNX1-regulated genes in lymphopoiesis using histone deacetylase inhibitors in ETV6/RUNX1-positive lymphoid leukemic cells. Clin Cancer Res Off J Am Assoc Cancer Res 13:1726–1735. doi:10.1158/1078-0432.CCR-06-2569
Stauber RH et al (2012) A combination of a ribonucleotide reductase inhibitor and histone deacetylase inhibitors downregulates EGFR and triggers BIM-dependent apoptosis in head and neck cancer. Oncotarget 3:31–43
Stempin S, Andres S, Scheer MB, Rode A, Nau H, Seidel A, Lampen A (2013) Valproic acid and its derivatives enhanced estrogenic activity but not androgenic activity in a structure dependent manner. Reprod Toxicol 42:49–57. doi:10.1016/j.reprotox.2013.07.019
Trtkova K, Paskova L, Matijescukova N, Kolar Z (2010) Formation of AR-SMRT binding in prostate cancer cells treated with natural histone deacetylase inhibitor. Cancer Biomark Sect A Dis Markers 7:79–90. doi:10.3233/CBM-2010-0150
Wang H et al (2016) Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3. Oncotarget. doi:10.18632/oncotarget.7888
Wieczorek M, Ginter T, Brand P, Heinzel T, Krämer OH (2012) Acetylation modulates the STAT signaling code. Cytokine Growth Factor Rev 23:293–305. doi:10.1016/j.cytogfr.2012.06.005
Yu H, Lee H, Herrmann A, Buettner R, Jove R (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14:736–746. doi:10.1038/nrc3818
Acknowledgments
We thank Christina Brachetti for excellent technical support. This study was mainly supported by grants to OHK from the Wilhelm Sander-Stiftung (#2010.078) and additionally by grants to OHK from the Deutsche Krebshilfe (#110909/#110125), the Deutsche Forschungsgemeinschaft (#KR2291/4-1/5-1), and intramural funding from the NMFZ Mainz and the University Medical Center Mainz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mahendrarajah, N., Paulus, R. & Krämer, O.H. Histone deacetylase inhibitors induce proteolysis of activated CDC42-associated kinase-1 in leukemic cells. J Cancer Res Clin Oncol 142, 2263–2273 (2016). https://doi.org/10.1007/s00432-016-2229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2229-x