Abstract
Background
Endocrine therapy is a mainstay of prostate cancer therapy. Given that few data exist on patient physician communication with regard to this field of therapy and adherence, we conducted a survey of patient members of a German support organization.
Patients and methods
We developed a structured questionnaire that was tested in a pilot version and then programmed as an online questionnaire.
Results
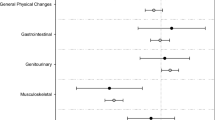
The questionnaire was completed by 694 patients. While 58 % of participants rated the information they received as comprehensive, 42 % did not. Fifty-one percentage stated that they were informed of side effects in detail, and 35 % received information on supportive treatments available in the event of side effects. Patients with higher education more often reported receiving information on side effects (p = 0.036) as well as alternatives for treatment (p = 0.001). Only 13 % stated that their questions were answered in detail, with 43 % receiving no answers or only non-detailed answers. Additional information was sought by 82 %, mostly from the Internet (67 %) and patient support groups (66 %). Seventy-six percentage experienced side effects that imposed limitations on their daily activities. Of those patients with side effects, 60 % reported that their physicians did not react to their complaints. There is a significant association between side effects in general and depression in particular and non-adherence (p < 0.01 and p = 0.002, respectively). In contrast, better information on side effects is associated with better adherence (p < 0001).
Conclusion
In order to improve adherence, detailed information on side effects and comprehensive supportive care is most important. Physicians should not rely on written information but should rather mainly engage in direct communication.

Similar content being viewed by others
References
Bell RJ, Fradkin P, Schwarz M, Davis SR (2013) Understanding discontinuation of oral adjuvant endocrine therapy by women with hormone receptor-positive invasive breast cancer nearly 4 years from diagnosis. Menopause 20(1):15–21
Bourke L, Kirkbridge P, Hooper R, Rosario AJ, Chico TJA, Rosario DJ (2013) Endocrine therapy in prostate cancer: time fr reappraisal of risks, benefits and cost-effectiveness? Brit J Cancer 108(9):13
Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R (2012) Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol 23:882–890
Gueth U, Myrick ME, Kilic N, Eppenberger-Castori S, Schmid SM (2012) Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Res Treat 131(2):491–499
Harle LK, Maggio M, Shahani S, Braga-Basaria M, Basaria S (2006) Endocrine complications of androgen-deprivation therapy in men with prostate cancer. Clin Adv Hematol Oncol 4(9):687–696
https://www.healthonnet.org/HONcode/German/. Assessed 27 July 2014
http://www.krebsgesellschaft.de/download/2009-pl-pca.pdf. Assessed 27 July 2014
http://www.krebsgesellschaft.de/download/patientenleitlinie_prostatakrebs_2_2013.pdf. Assessed 27 July 2014
http://www.discern.org.uk/discern_instrument.php. Assessed 27 July 2014
Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms; Langversion 3.1–2. Aktualisierung, Oktober 2014; AWMF-RegisterNummer 043/022OL
Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD (2006) Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev 4:CD006019
Liu Y, Malin JL, Diamant AL, Thind A, Maly RC (2013) Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat 137:829–836
Mottet N, Peneau M, Mzeron JJ, Molinie V, Richaud P (2012) Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur Urol 62(2):213–219
Nair B, Wilt T, MacDonald R, Rutks I (2002) Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev 1:CD003506
Payne H, Mason M (2011) Androgen deprivation therapy as adjuvant/neoadjuvant to radiotherapy for high-risk localised and locally advanced prostate cancer: recent developments. Br J Cancer 105(11):1628–1634
Steckelberg A, Berger B, Köpke S, Heesen C, Muehlhauser I (2005) Kriterien für evidenzbasierte Patienteninformationen. Z ärztl Fortb Qualitätssich 99:343–351
Studer U, Whelan P, Albrecht W, Casselman J, Reijke TMD, Knoenageö H, Madersbacher S, Isorna S, Sundaram SK, Collette L (2011) Long term results of immediate versus deferred deprivation in patients with no local treatment for T0-4 N0-2 M0 prostate cancer (EORTC 30891). J Urol 185(4S):e144
Widmarl A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, Lund JA, Tasdemir I, Hoyer M, Wiklund F, Fossa SD (2009) Endocrine treatment with or without radiotherapy, in locally advances prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373(9660):301–308
Wuensch P, Hahne A, Haidinger R, Meißler K, Tenter B, Stoll C, Senf B, Huebner J (2015) Discontinuation and non-adherence to endocrine therapy in breast cancer patients: is lack of communication the decisive factor? J Cancer Res Clin Oncol 141(1):55–60
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do have no conflicts of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. According to the regulations of the ethics committee of the University Hospital Frankfurt/Main for anonymous surveys, no ethics evaluation was necessary. As it was an anonymous survey, no written informed consent was obtained from the participants who voluntarily took part.
Rights and permissions
About this article
Cite this article
Jung, B., Stoll, C., Feick, G. et al. Prostate cancer patients’ report on communication about endocrine therapy and its association with adherence. J Cancer Res Clin Oncol 142, 465–470 (2016). https://doi.org/10.1007/s00432-015-2059-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2059-2