Abstract
Introduction
Testing for the epidermal growth factor receptor (EGFR) gene mutations requires considerable multidisciplinary experience of clinicians (for appropriate patient selection), pathologists (for selection of appropriate cytological or histological material) and geneticists (for performing and reporting reliable molecular tests). We present our experience on the efficacy of routine EGFR testing in various types of tumor samples and the frequency of EGFR mutations in a large series of Polish non-small cell lung cancer (NSCLC) patients.
Methods
Deletions in exon 19 and substitution L858R in exon 21 of EGFR gene were assessed using real-time PCR techniques in 1,138 small biopsies or cytological specimens and in 1,312 surgical samples.
Results
Out of 2,450 diagnostic samples (containing >10 % of tumor cells), the occurrence of EGFR gene mutations was 9 %; more frequently in women (13.9 %) and adenocarcinoma patients (10 %), particularly with accompanying expression of TTF1 (13.0 %). The frequency of EGFR gene mutations was similar in cytological and histological specimens, and in primary and metastatic lesions, and did not depend on the percentage of tumor cells and quality of isolated DNA. Cytological or small biopsy, compared to surgical specimens showed lower percentage of tumor cells, with no impact on the quality of real-time PCR assay.
Conclusion
Cytological and small biopsy samples with low (10–20 %) content of tumor cells and specimens from metastatic lesions are a sufficient source for EGFR mutation testing in NSCLC patients. The incidence of EGFR gene mutations in examined population was similar to those reported in other Caucasian populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Appropriate epidermal growth factor receptor (EGFR) gene mutation testing is an essential element in selecting non-small cell lung cancer (NSCLC) patients for therapy with tyrosine kinase inhibitors of EGFR (TKIs EGFR). Erlotinib and gefitinib (reversible TKIs EGFR) as well as afatinib (irreversible TKI EGFR) constitute effective first- and subsequent line management in advanced NSCLC patients with EGFR mutations. A series of phase III clinical trials showed higher response rates and longer progression-free survival in patients with EGFR mutations treated with first-line EGFR TKIs compared to platinum-based chemotherapy (Petrelli et al. 2012; Lee et al. 2013). Further, recent meta-analyses demonstrated the effectiveness of EGFR TKIs in second-line treatment in this subset of patients (Petrelli et al. 2012; Lee et al. 2013). Currently, there is no strong clinical and economic rationale for using EGFR TKIs in first- or second-line therapy in patients with wild-type EGFR. The activating EGFR mutations are the only independent predictors of TKIs EGFR activity. Although EGFR mutations occur more frequently in females, non-smokers and East-Asian patients, these factors should not be used for selection of patients for EGFR TKIs therapy (Petrelli et al. 2012; Lee et al. 2013; Travis et al. 2011). In many countries, EGFR mutational analysis is performed exclusively in patients with adenocarcinoma (AC) or adenosquamous carcinoma (ADSQ) in whom the EGFR gene mutations occur more frequently than in other NSCLC types. Higher incidence of EGFR mutations in lepidic non-mucinous, papillary and solid AC compared to invasive mucinous and acinar AC, as well as in tumors expressing thyroid transcription factor 1 (TTF1) is not well understood (Aisner and Marshall 2012; Eberhard et al. 2008).
EGFR mutations occur in approximately 10 % of Caucasian NSCLC patients. Deletions in exon 19 and substitution L858R in exon 21 account for approximately 90 % of EGFR mutations (Aisner and Marshall 2012; Eberhard et al. 2008; Rosell et al. 2009, 2012; Boch et al. 2013; Gahr et al. 2013). The rare EGFR mutations are scattered in exons 18–21 of tyrosine kinase domain of the EGFR gene. There are large discrepancies in the occurence of these mutations between particular clinical centers (Rosell et al. 2009, 2012; Boch et al. 2013; Gahr et al. 2013). This is mostly due to differences in selection of NSCLC patients for EGFR testing (patients with non-AC, patients with early stage NSCLC who underwent surgery, etc.). Another cause of these differences is most likely technical issues related to genotyping and the sensitivity of particular molecular methods may influence the effectiveness of EGFR. Direct sequencing technique requires more than 50 % of tumor cells in the specimen (e.g., surgical material), whereas real-time PCR and next-generation sequencing techniques (CE-IVD methods) allow EGFR detection in samples containing as few as 1 % tumor cells. Despite this, EGFR testing in low-cellularity specimens (small biopsies, cytological smears) is still being questioned (Aisner and Marshall 2012; Eberhard et al. 2008). Additionally, thermal and chemical treatment of cells or tissue specimens (formalin fixation and paraffin embedding) reduces the quantity and quality of DNA. In consequence in a proportion of cases EGFR mutations may be missed (Aisner and Marshall 2012; Eberhard et al. 2008).
The aim of this retrospective study was to assess the relationship between the type of examined material and the efficacy of EGFR testing in a large series of Polish NSCLC patients subjected to routine screening for TKIs EGFR therapy. We also estimated in this population the frequency of deletion in exon 19 and L858R substitution in exon 21 of the EGFR gene.
Materials and methods
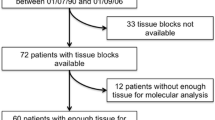
A total of 2,450 NSCLC patients (median age: 63 ± 9 years), including 1,503 males and 947 females from 10 Polish cancer centers were referred for routine EGFR testing during selection for EGFR TKIs therapy (Table 1). The type of NSCLC tumors was diagnosed by standard hematoxylin and eosin staining, and in the case of scarce material—by immunohistochemistry. Expression of TTF1 was assessed in 727 patients, p63 in 290, CK7 in 465 and CK5/6 in 192 patients. Surgical tissue samples from primary tumors, distant metastases and involved lymph nodes were collected from 1,138 patients, and small biopsy or cytological samples from 1,312 patients (Table 2).
The molecular testing was performed in four genetic laboratories (Lublin, Warsaw, Gdansk and Bydgoszcz). DNA was isolated from formalin-fixed paraffin-embedded (FFPE) tissue sections or cellblock sections using QIAamp DNA FFPE Tissue Kit (Qiagen, Canada) and Cobas DNA Sample Preparation Kit (Roche, USA) in accordance with the manufacturer’s instruction. DNA from mechanically disrupted fresh frozen tissues and DNA from scrapped materials of tumor cells marked by pathologists in cytological smears were isolated using QIAamp DNA Mini Kit (Qiagen, Canada). The data on DNA concentration measured with spectrophotometry were available for 1,078 cases and were predetermined before EGFR testing. An A260/A280 ratio of 1.8–2 was considered as a pure and well quality of DNA and such DNA was qualified for EGFR testing. The late amplification plot of internal control (after 35 cycles) was defined as a “weak amplification” of internal control or positive sample in real-time PCR method.
EGFR Mutation Analysis Kit (Entrogen, USA) and real-time PCR technique were used for EGFR mutation analysis in 1,882 samples in Lublin and Bydgoszcz laboratories, whereas Cobas EGFR Mutation Test (Roche, USA) in 147 samples in Gdansk. Laboratory-defined assay and PNA–LNA PCR Clamp method in real-time PCR technique were applied to analyze EGFR gene mutation in 421 samples from Warsaw. All laboratories participated in External Quality Assessment (EQA) scheme for molecular genetic analysis of EGFR in non-small cell lung cancer organized by European Molecular Quality Network (EMQN). Despite of various methods using in individual laboratories, the percentage of patients with positive EGFR status was similar (insignificant differences). Hence, single technique (real-time PCR) was used for evaluation of EGFR mutations in all Centers.
Statistical analysis was performed using Statistica, version 10. Associations between EGFR mutations, clinical factors and the type of tumor material were examined using chi-square test. The Student’s t test was used for testing equality of population medians among groups. p values below 0.05 were considered significant.
The study was approved by the Ethical Committee of the coordinating center, the Medical University in Lublin (decision no. KE-0254/131/2011).
Results
Activating mutations of EGFR gene were found in 9 % of NSCLC tumors and included deletion in exon 19 in 123 cases and L858R substitution in exon 21 in 98 cases (Table 2). The frequency of rare EGFR mutations was not shown due to differences in the type of examined rare mutations between centers involved in the study. EGFR mutations were observed significantly more frequently in women compared with men (p < 0.0001). There was a similar percentage of patients with EGFR mutations in younger (<63 years) compared to older patients and a median age of patients with and without EGFR mutations was exactly the same (63 years). EGFR mutations occurred more frequently in AC compared with other NSCLC types (p = 0.0008) and with not otherwise specified (NOS) NSCLC (p = 0.0007). However, EGFR mutations were found in all types of NSCLC and were surprisingly frequent in ADSQ (19.1 %). The feasibility of EGFR testing was similar in different types of material, and in samples from primary versus metastatic tumors. The mutations were slightly more frequent in surgical (9.8 %) compared to small biopsy and cytological specimens (8.3 %) (p = 0.211). EGFR mutations were significantly more frequent (p = 0.04) in surgically resected primary tumors (10.6 %) than in metastatic lymph nodes obtained by mediastinoscopy or endobronchial ultrasound transbronchial aspiration (7.7 %). The proportion between exon 19 deletion and exon 21 L858R substitution was independent of gender, age, NSCLC histology and the type of examined material (Table 2).
The expression of TTF1 antigen in non-squamous NSCLC was significantly more frequent (p = 0.043) in females (82.9 %) than in males (76.9 %) and did not depend on patients’ age. EGFR gene mutations were more frequent (p = 0.049) in TTF1-positive compared with TTF1-negative tumors (Table 3). The expression of other cancer tissue markers (CK7, CK5/6, p63) did not correlate with the occurrence of EGFR mutations.
Most of molecular tests were performed on material containing ≥20 % of tumor cells (84.9 %) and with good amplification of positive internal control in real-time PCR assay (77.1 %; Table 4). However, EGFR mutations were detected with similar frequency (p = 0.495) in samples containing ≥20 and <20 % of cancer cells. EGFR mutations were found in 191 (9.2 %) cases out of 2,079 samples with ≥20 % tumor cells and in 30 (8.1 %) cases out of 371 samples of <20 % tumor cells. EGFR mutations were found in 9.5 and 7.5 % of cases with good and weak amplification of internal positive control in real-time PCR, respectively (p = 0.145). The total concentration of isolated DNA was 56.4 ± 226 ng/μl. DNA concentrations were similar (p = 0.54) in patients with wild-type and mutated EGFR gene (57.4 ± 228.18 and 51 ± 202.93 ng/μl, respectively).
Scant cellularity was more frequent in biopsy specimens compared to surgically resected tissues (p = 0.001). Low content of tumor cells were most common in specimens from transthoracic biopsies and endobronchial ultrasonography-guided transbronchial needle aspiration biopsy (EBUS-TBNA). Sufficient PCR cycles number for amplification of internal positive control were similar in both surgical and biopsy specimens. However, a weak amplification of internal positive control was most common in surgically resected primary tumors and in EBUS-TBNA biopsies. Biopsy specimens, compared to surgically resected material contained significantly lower DNA concentration (p < 0.0001) (Table 4).
Discussion
EGFR mutations occur more frequently in females, non-smokers and ACs (Petrelli et al. 2012; Lee et al. 2013; Rosell et al. 2009). The frequency of reported EGFR gene mutations in the European AC patients ranges from 8.7 % in Germany to 17.8 % in Spain (Rosell et al. 2009; Boch et al. 2013; Gahr et al. 2013). In these populations, the frequency of EGFR mutations in large cell carcinoma (LCC) patients was 11.5 and 3.6 %, respectively. The frequency of EGFR gene mutations in German squamous cell carcinoma (SCC) patients ranged from 0 to 3.5 % (Boch et al. 2013; Gahr et al. 2013). The frequency of L858R substitution was similar to the frequency of EGFR gene deletions. In the Spanish (AC and LCC) and German (AC only) populations, the EGFR gene mutations were more common in females (30 and 14 %, respectively) than in males (8.2 and 1.2 %, respectively) (Rosell et al. 2009; Boch et al. 2013). In the Spanish population, the percentage of EGFR mutations was independent of age: 13.9 and 18.9 % in patients below and above 56.7 years, respectively (Rosell et al. 2009). The differences between the frequencies of mutations in particular populations were mainly due to various criteria for selecting patients for EGFR testing, e.g., considering smoking history.
Our study includes the largest series of European NSCLC patients tested for EGFR mutations. The frequency of EGFR mutations (9 % in the entire population, 14 % in females and 10 % in AC) in patients considered to TKIs EGFR therapy was similar to that reported in the German population (Boch et al. 2013). Notably, we found a relatively high percentage of EGFR mutations in ADSQ (19.1 %) and in SCC (5.2 %) patients. It is likely that some SCC patients might have been misdiagnosed, as immunohistochemistry was not routinely performed. Moreover, the data on patient smoking status were not available in our analysis. Also, the weakness of our study results from the retrospective character of analysis.
There is an ongoing debate on whether the expression of immunomarkers, e.g., TTF1 on tumor cells could help to predict the EGFR mutations in AC patients. In Japanese AC patients, TTF-1 expression was found to be related to never smoking status and to the presence of EGFR mutations (Hiramatsu et al. 2010). The frequency of EGFR mutations in this study was 58 % in the entire group and 65 % in the TTF1-positive subset. In the German population, the occurrence of EGFR mutations was associated with TTF1 positivity and almost all EGFR-mutated NSCLC patients were TTF-1 positive (Tapia et al. 2009). Similar results were obtained by Leary et al. (Leary et al. 2012) who ascertained that all examined tumors with EGFR mutations were non-mucinous TTF1-positive. These three studies combined involved fewer than 500 patients. The results of our study including 727 patients confirmed these findings. However, in our material, EGFR mutations were also found in TTF1-negative tumors, suggesting that TTF1 negativity should not exclude patients from EGFR testing (Table 3).
There are only a few studies evaluating the presence of EGFR mutations in primary NSCLC and in corresponding metastases. In the study of Togashi et al. (2011), lung cancer metastases were diagnosed less frequently in wild-type EGFR compared to EGFR-mutated AC. However, in this study, the information about type of tumor samples (primary or metastatic) used for EGFR mutation analysis was not provided (Togashi et al. 2011). Sun et al. (2011), in a study including 80 patients showed full concordance of EGFR mutational status between 21 primary tumors and corresponding 26 lymph node metastases (Sun et al. 2011). We earlier found only 6.3 % (9/143) of EGFR gene mutations in the brain NSCLC metastases, with the full concordance with mutational status of primary tumor (Wojas-Krawczyk et al. 2013). In the current study, the frequency of EGFR mutations was similar in the primary and metastatic sites including lymph nodes (Table 2). This indicates that molecular testing may be reliably performed on both primary and metastatic tumors, depending on their availability.
It has been postulated that testing for EGFR mutations should use histological rather than cytological material (Billah et al. 2011; Smouse et al. 2009). Billah et al. (2011) concluded that 6.2 % (13/209) of cytological specimens (including 99 EBUS-TBNA specimens) were insufficient for molecular diagnosis, but they did not compare their results with corresponding surgical material. Moreover, the percentage of EGFR mutations in this series was relatively high (20 %). Smouse et al. (2009) reported the failure of molecular diagnosis in 3.5 % (8/227) and 8.3 % (1/12) of surgical and cytological specimens, respectively. However, the frequency of EGFR mutations was higher in cytological compared to surgical specimens (58 and 28 %, respectively). Esterbrook et al. (2013) showed that 11.2 % (4/36) of EBUS-TBNA samples were ineligible for EGFR mutation testing. Khode et al. (2013) found EGFR mutations in 28.6 % (16/56) of FFPE samples from resected tissues and biopsy specimens and in only 14.3 % (9/63) of cytological smears. This study also showed a high concordance of results obtained from cytological smears and FFPE samples achieved from the same anatomic sites. Additionally, the sensitivity of real-time PCR was lower than that of pyrosequencing platform (13 and 25 detected mutations, respectively). In a similar study, the overall EGFR mutation concordance between 60 histologic and corresponding cytological specimens was 91.7 % (Sun et al. 2013). More recently, Ma et al. (2012) tested EGFR mutations in 269 cytological specimens and in 1,141 surgically resected tissues from the Asian patients. EGFR mutations were found in 39 and 48 % of cytological and tissue specimens, respectively. An Italian study showed 9.7 % of EGFR mutations in fresh specimens obtained during CT-guided aspiration biopsy of peripheral AC (Stella et al. 2013). In another Italian study, including 92 cytological specimens, mutations were found in 24 % of the cases, and 12 % of samples were not assessable (Allegrini et al. 2012). All these studies were relatively small and used various laboratory techniques (e.g., macro- or microdissection) of DNA preparation and molecular testing. Our preliminary study (Krawczyk et al. 2012) (460 NSCLC patients) showed insignificantly higher in frequency of EGFR gene mutations in tissue (12.4 %) compared to cytological specimens (8.8 %). In another large study (755 NSCLC patients, EGFR testing was less successful in samples with low (<2 ng/μl) compared to those with high DNA concentration (70 vs. 96 %, respectively), with the corresponding EGFR positivity of 13 and 9.2 %, respectively (Leary et al. 2012).
It is generally believed that the quantity and quality of the material are crucial to perform a reliable EGFR mutation analysis. However, in the current study, including large series of routinely assayed NSCLC patients, EGFR mutations were detected with similar frequency in surgical and biopsy specimens, as well as in primary and metastatic sites. Further, the low percentage of tumor cells did not preclude effective EGFR analysis using real-time PCR techniques. Despite significant differences in tumor cellularity, the quality (amplification of internal positive control in real-time PCR) and the quantity of DNA obtained from different types of materials was similar, and so was the DNA concentration in samples with and without EGFR mutations. Our results confirm therefore that samples from small biopsies or cytology allow for reliable EGFR mutation testing.
References
Aisner DL, Marshall CB (2012) Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol 138:332–346
Allegrini S, Antona J, Mezzapelle R et al (2012) Epidermal growth factor receptor gene analysis with a highly sensitive molecular assay in routine cytologic specimens of lung adenocarcinoma. Am J Clin Pathol 138:377–381
Billah S, Stewart J, Staerkel G et al (2011) EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol 119:111–117
Boch C, Kollmeier J, Roth A et al (2013) The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open 3:e002560
Eberhard DA, Giaccone G, Johnson BE et al (2008) Biomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol 26:983–994
Esterbrook G, Anathhanam S, Plant PK (2013) Adequacy of endobronchial ultrasound transbronchial needle aspiration samples in the subtyping of non-small cell lung cancer. Lung Cancer 80:30–34
Gahr S, Stoehr R, Geissinger E et al (2013) EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice. Br J Cancer 109:1281–1288
Hiramatsu M, Ninomiya H, Inamura K et al (2010) Activation status of receptor tyrosine kinase downstream pathways in primary lung adenocarcinoma with reference of KRAS and EGFR mutations. Lung Cancer 70:94–102
Khode R, Larsen DA, Culbreath BC et al (2013) Comparative study of epidermal growth factor receptor mutation analysis on cytology smears and surgical pathology specimens from primary and metastatic lung carcinomas. Cancer Cytopathol 121:361–369
Krawczyk P, Ramlau R, Powrózek T et al (2012) The detection of EGFR mutations in patients with non-small cell lung cancer in selected molecular diagnostics centers in Poland. Kardiochir Torakochir Pol 9:431–438
Leary AF, Castro DG, Nicholson AG et al (2012) Establishing an EGFR mutation screening service for non-small cell lung cancer—sample quality criteria and candidate histological predictors. Eur J Cancer 48:61–67
Lee CK, Brown C, Grala RJ et al (2013) Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 105:595–605
Ma ES, Ng WK, Wong CL (2012) EGFR gene mutation study in cytology specimens. Acta Cytol 56:661–668
Petrelli F, Borgonovo K, Cabiddu M et al (2012) Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer: a meta-analysis of 13 randomised trials. Clin Lung Cancer 13:107–114
Rosell R, Moran T, Queralt C et al (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361:958–967
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Smouse JH, Cibas ES, Janne PA et al (2009) EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer Cytopathol 117:67–72
Stella GM, Scabini R, Inghilleri S et al (2013) EGFR and KRAS mutational profiling in fresh non-small cell lung cancer (NSCLC) cells. J Cancer Res Clin Oncol 139:1327–1335
Sun L, Zhang Q, Luan H et al (2011) Comparison of KRAS and EGFR gene status between primary non-small cell lung cancer and local lymph node metastases: implications for clinical practice. J Exp Clin Cancer Res 30:30–38
Sun PL, Jin Y, Kim H et al (2013) High concordance of EGFR mutation status between histologic and corresponding cytologic specimens of lung adenocarcinomas. Cancer Cytopathol 121:311–319
Tapia C, Savic S, Bihl M et al (2009) EGFR mutation analysis in non-small-cell lung cancer: experience from routine diagnostics. Pathologe 30:384–392
Togashi Y, Masago K, Kubo T et al (2011) Association of diffuse, random pulmonary metastases, including milliary metastases, with epidermal growth factor receptor mutations in lung adenocarcinoma. Cancer 117:819–825
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 6:244–285
Wojas-Krawczyk K, Skroński M, Krawczyk P et al (2013) EGFR activating mutations detected by different PCR techniques in Caucasian NSCLC patients with CNS metastases: short report. Clin Exp Metastasis. doi:10.1007/s10585-013-9603-8
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Krawczyk, P., Ramlau, R., Chorostowska-Wynimko, J. et al. The efficacy of EGFR gene mutation testing in various samples from non-small cell lung cancer patients: a multicenter retrospective study. J Cancer Res Clin Oncol 141, 61–68 (2015). https://doi.org/10.1007/s00432-014-1789-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1789-x