Abstract
Purpose
We aimed to compare the treatment efficacy of cetuximab versus bevacizumab in combination with either irinotecan-based or oxaliplatin-based regimens (targeted triplet) as the first-line treatment for patients with metastatic colorectal cancer.
Methods
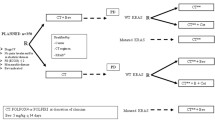
Between April 2005 and March 2012, patients (n = 158) diagnosed with metastatic colorectal cancer after at least four courses of first-line bevacizumab-based (n = 95) or cetuximab-based triplet (n = 63) were retrospectively analyzed. The KRAS genotypes were sequenced for all patients. The Kaplan–Meier method was used for survival analysis, and Cox proportional hazards models were used for univariate and multivariate analyses.
Results
Cetuximab-based triplet was associated with a higher objective response rate (66.0 vs. 47.2 %, p = 0.037) and a higher conversion rate to resectability (39.7 vs. 20.0 %, p = 0.007) compared to bevacizumab-based triplet. Compared with bevacizumab-based triplet, cetuximab-based triplet significantly increased progression-free survival in patients with measurable metastatic colorectal cancer who achieved objective tumor response (responders) (median 13.1 vs. 10.5 months, p = 0.023), but no significant increase was observed for overall survival. After adjustment for group differences in baseline characteristics and combined chemotherapy agents, cetuximab-based triplet remained an independent determinant of progression-free survival in responders as compared with bevacizumab-based triplet. KRAS mutation was not a prognostic factor in patients with metastatic colorectal cancer.
Conclusions
As compared with bevacizumab-based triplet, cetuximab-based triplet as the first-line treatment of metastatic colorectal cancer was associated with better progression-free survival in patients with measurable tumors who achieved objective tumor response to bio-chemotherapy.


Similar content being viewed by others
References
Abdelwahab S, Azmy A, Abdel-Aziz H, Salim H, Mahmoud A (2012) Anti-EGFR (cetuximab) combined with irinotecan for treatment of elderly patients with metastatic colorectal cancer (mCRC). J Cancer Res Clin Oncol 138(9):1487–1492. doi:10.1007/s00432-012-1229-8
Baldus SE, Schaefer KL, Engers R, Hartleb D, Stoecklein NH, Gabbert HE (2010) Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res 16(3):790–799. doi:10.1158/1078-0432.CCR-09-2446
Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ, Fuchs CS, Grem JL, Hunt S, Kamel A, Leong LA, Lin E, May KS, Mulcahy MF, Murphy K, Rohren E, Ryan DP, Saltz L, Sharma S, Shibata D, Skibber JM, Small W Jr, Sofocleous CT, Venook AP, Willett CG, Gregory KM, Freedman-Cass DA (2013) Metastatic colon cancer, version 3. 2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 11(2):141–152; quiz 152
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27(5):663–671. doi:10.1200/jco.2008.20.8397
Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Kohne CH (2012) Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer (Oxford, England: 1990) 48(10):1466–1475. doi:10.1016/j.ejca.2012.02.057
Bouchahda M, Macarulla T, Liedo G, Levi F, Elez ME, Paule B, Karaboue A, Artru P, Tabernero J, Machover D, Innominato P, Goldschmidt E, Bonnet D, Ducreux M, Castagne V, Guimbaud R (2011) Feasibility of cetuximab given with a simplified schedule every 2 weeks in advanced colorectal cancer: a multicenter, retrospective analysis. Med Oncol 28(Suppl 1):S253–S258. doi:10.1007/s12032-010-9716-8
Brodowicz T, Ciuleanu TE, Radosavljevic D, Shacham-Shmueli E, Vrbanec D, Plate S, Mrsic-Krmpotic Z, Dank M, Purkalne G, Messinger D, Zielinski CC (2013) FOLFOX4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with KRAS wild-type metastatic colorectal cancer: a randomized phase II CECOG study. Ann Oncol. doi:10.1093/annonc/mdt116
Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, Dinniwell R, Brierley J, Kavanagh BD, Dawson LA, Schefter TE (2011) Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 117(17):4060–4069. doi:10.1002/cncr.25997
Diaz-Rubio E, Gomez-Espana A, Massuti B, Sastre J, Reboredo M, Manzano JL, Rivera F, Safont MJ, Montagut C, Gonzalez E, Benavides M, Marcuello E, Cervantes A, Martinez de Prado P, Fernandez-Martos C, Arrivi A, Bando I, Aranda E, Spanish Cooperative Group for the Treatment of Digestive T (2012) Role of Kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative study. PLoS ONE 7(10):e47345. doi:10.1371/journal.pone.0047345
Eklof V, Wikberg ML, Edin S, Dahlin AM, Jonsson BA, Oberg A, Rutegard J, Palmqvist R (2013) The prognostic role of KRAS, BRAF, PIK3CA and PTEN in colorectal cancer. Br J Cancer 108(10):2153–2163. doi:10.1038/bjc.2013.212
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Kohne CH (2010) Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 11(1):38–47. doi:10.1016/S1470-2045(09)70330-4
Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ (2012) Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 30(3):263–267. doi:10.1200/JCO.2011.37.1039
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25(30):4779–4786. doi:10.1200/jco.2007.11.3357
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22(7):1209–1214. doi:10.1200/JCO.2004.11.037
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, Heintges T, Lerchenmueller J, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Mueller S, Schaefer B, Modest DP, Jung A, Stintzing S (2013) Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3). J Clin Oncol 31(Suppl):abstr LBA3506
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O (2009) The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 14(1):22–28. doi:10.1634/theoncologist.2008-0213
Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, Hurwitz HI, Kabbinavar F, Novotny WF, Hillan KJ, Koeppen H (2005) Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst 97(13):981–989. doi:10.1093/jnci/dji174
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765. doi:10.1056/NEJMoa0804385
Kemeny NE, Jarnagin WR, Capanu M, Fong Y, Gewirtz AN, Dematteo RP, D’Angelica MI (2011) Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol 29(7):884–889. doi:10.1200/JCO.2010.32.5977
Klaver YL, Simkens LH, Lemmens VE, Koopman M, Teerenstra S, Bleichrodt RP, de Hingh IH, Punt CJ (2012) Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol 38(7):617–623. doi:10.1016/j.ejso.2012.03.008
Macedo LT, da Costa Lima AB, Sasse AD (2012) Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer 12:89. doi:10.1186/1471-2407-12-89
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP (2011) Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377(9783):2103–2114. doi:10.1016/s0140-6736(11)60613-2
Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J (2009) Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer 101(7):1033–1038. doi:10.1038/sj.bjc.6605259
Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, Poston G (2011) Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 13(9):e252–e265. doi:10.1111/j.1463-1318.2011.02695.x
Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J (2013) Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol 31(6):759–765. doi:10.1200/JCO.2012.45.1492
Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S (2011) Cetuximab and panitumumab in KRAS wild-type colorectal cancer: a meta-analysis. Int J Colorectal Dis 26(7):823–833. doi:10.1007/s00384-011-1149-0
Price TJ, Hardingham JE, Lee CK, Weickhardt A, Townsend AR, Wrin JW, Chua A, Shivasami A, Cummins MM, Murone C, Tebbutt NC (2011) Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol 29(19):2675–2682. doi:10.1200/JCO.2010.34.5520
Primrose JN, Falk S, Finch-Jones M, Valle JW, Sherlock D, Hornbuckle J, Gardner-Thorpe J, Smith D, Imber C, Hickish T, Davidson B, Cunningham D, Poston GJ, Maughan T, Rees M, Stanton L, Little L, Bowers M, Wood W, Bridgewater JA (2013) A randomized clinical trial of chemotherapy compared to chemotherapy in combination with cetuximab in k-RAS wild-type patients with operable metastases from colorectal cancer: The new EPOC study. J Clin Oncol 31(Suppl):abstr 3504
Ramanathan RK (2008) Alternative dosing schedules for cetuximab: a role for biweekly administration? Clin Colorectal Cancer 7(6):364–368. doi:10.3816/CCC.2008.n.048
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. doi:10.1200/jco.2007.14.9930
Teng HW, Huang YC, Lin JK, Chen WS, Lin TC, Jiang JK, Yen CC, Li AF, Wang HW, Chang SC, Lan YT, Lin CC, Wang HS, Yang SH (2012) BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol 106(2):123–129. doi:10.1002/jso.23063
Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, Parmar MK, Meade AM (2012) Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev 38(6):618–625. doi:10.1016/j.ctrv.2011.11.002
Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, Ychou M, Rougier P, European Colorectal Metastases Treatment G (2006) Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer (Oxford, England: 1990) 42(14):2212–2221. doi:10.1016/j.ejca.2006.04.012
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360(14):1408–1417. doi:10.1056/NEJMoa0805019
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019. doi:10.1200/jco.2010.33.5091
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY, Xu J (2013) Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 31(16):1931–1938. doi:10.1200/JCO.2012.44.8308
Acknowledgments
The authors thank the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital, and the Taipei Veterans General Hospital, Taiwan Clinical Oncology Research Foundation, Department of Health, Taiwan, for their support for our work.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2014_1741_MOESM1_ESM.tif
aObjective tumor response and b the intensity of objective tumor response (26.4 % strong versus 39.6 % weak) in the cetuximab group. c Objective response and d the intensity of objective tumor response (8.3 % strong versus 38.9 % weak) in the bevacizumab group. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease (TIFF 16793 kb)
432_2014_1741_MOESM2_ESM.tif
aProgression-free survival (PFS) and b overall survival (OS) of patients with non-measurable tumors, according to targeted agents. c PFS and d OS of patients with stable plus progressive disease according to targeted agents. SD, stable disease; PD, progressive disease (TIFF 16584 kb)
432_2014_1741_MOESM3_ESM.tif
aProgression-free survival of all patients according to metastasectomy status. b Overall survival of all patients according to metastasectomy status. HR, hazard ratio (TIFF 7255 kb)
Rights and permissions
About this article
Cite this article
Yang, YH., Lin, JK., Chen, WS. et al. Comparison of cetuximab to bevacizumab as the first-line bio-chemotherapy for patients with metastatic colorectal cancer: Superior progression-free survival is restricted to patients with measurable tumors and objective tumor response—a retrospective study. J Cancer Res Clin Oncol 140, 1927–1936 (2014). https://doi.org/10.1007/s00432-014-1741-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1741-0