Abstract
Background
Preoperative or neoadjuvant chemotherapy is an option in patients with large operable breast cancer to facilitate the breast conservation and to downstage the disease to make inoperable breast cancer to operable one. It is also called the window of opportunity; it provides a unique opportunity to derive biological information related to tumor response. Neoadjuvant chemotherapy has been compared with standard, postoperative adjuvant chemotherapy with goals of improving survival and facilitating local therapies. Unfortunately, neoadjuvant chemotherapy does not seem to improve overall survival. There is a lack of data from India regarding the neoadjuvant chemotherapy. The present study was carried out to assess the response to neoadjuvant chemotherapy in breast cancer.
Materials and methods
We retrospectively analyzed the records of patients who were started on neoadjuvant chemotherapy (NACT) at our center for 1 year (August 2012 to July 2013). Case files were thoroughly reviewed, and patient’s characteristics (age, pre-/postmenopausal status, family history of breast/ovarian/other cancer), mode of detection, treatment, and histological features were analyzed.
Results
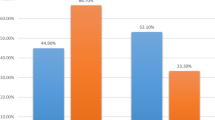
Out of 322 patients with breast cancer registered in our institute, 80 patients received neoadjuvant chemotherapy. Median age was 45 years. The most common presentation was left-sided breast lump (Lt > Rt) with a median duration of symptoms was 4 months. Postmenopausal patients (53.75 %) were more than premenopausal (46.25 %). Seventy-two patients were stage III and 8 were stage II disease. Bilateral breast cancer was seen in 8 patients. Most common histological type was invasive ductal carcinoma (95 %). Estrogen receptor (ER) and/or progesterone (PR) positive were seen in 47 (58.75 %) patients. Ten patients were HER2 positive and ER/PR negative, and 5 patients were triple positive. Triple-negative patients were 22 (27.5 %). The most common neoadjuvant chemotherapy protocol used was FEC. Clinical response before surgery was CR 13 %, PR 68.68 %, stable disease 11.62 %, and progressive disease 4.65 %. Pathological CR was seen in 6.9 % of tumors. Nodal status at surgery was ypN0-40 %, ypN1-28. 5 %. ypN2-27 %, and ypN3-4.28 %.
Conclusion
In a population of predominantly locally advanced patients, NACT with anthracyclines yielded pCR rates comparable to published studies. There were a high proportion of HER2-positive patients, most of whom could not receive anti-HER2 therapy due to financial reasons.




Similar content being viewed by others
References
Baldini E, Gardin G, Giannessi PG, Evangelista G, Roncella M, Prochilo T et al (2003) Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: a randomized phase III trial in locally advanced breast cancer. Ann Oncol 14:227–232
Charehbili A, Fontein DB, Kroep JR et al (2014) Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: a systematic review. Cancer Treat Rev 40:86–92
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366:2087–2106
Cortazar P, Zhang L, Untch M, Mehta K, Costantino J, Wolmark N et al (2012) Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC). Cancer Res 72(24 Suppl):S1–S11; Abstract nr
Danforth DN Jr, Lippman ME, McDonald H et al (1990) Effect of preoperative chemotherapy on mastectomy for locally advanced breast cancer. Am Surg 56:6–11
Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M et al (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707–1716
Deo SV, Bhutani M, Shukla NK, Raina V, Rath GK, Purkayasth J (2003) Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). J Surg Oncol 84(4):192–197
Erol K, Baltali E, Altundag K, Guler N, Ozisik Y, Onat DA et al (2005) Neoadjuvant chemotherapy with cyclophosphamide, mitoxantrone, and 5-fluorouracil in locally advanced breast cancer. Onkologie 28:81–85
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483–2493
Ghosh I, Dabkara D, Dwary A, Ahmed R (2013) Neoadjuvant Chemotherapy (NAC) for breast cancer—preliminary experience from a tertiary cancer center in India. Ann Oncol 24(Supplement 3):19–22. doi:10.1093/annonc/mdt081.8
Gralow JR, Burstein HJ, Wood W et al (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26:814–819
Gupta S, Bharat R, Shet T, Desai SB, Patil VM, Bakshi A, Parmar V, Badwe RA (2012) Single agent weekly paclitaxel as neoadjuvant chemotherapy in locally advanced breast cancer: a feasibility study. Clin Oncol 24:604–609
Hortobagyi GN, Ames FC, Buzdar AU et al (1988) Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 62:2507–2516
Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P et al (2006) Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol 24:1831–1838
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K et al (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17:460–469
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. JCO 26(8):1275–1281
Patel T, Gupta A, Shah M (2013) Pathological predictive factors for tumor response in locally advanced breast carcinomas treated with anthracycline-based neoadjuvant chemotherapy. J Cancer Res Ther 9:245–249
Raina V, Kunjahari M, Shukla NK, Deo S, Sharma A, Mohanti BK, Sharma DN (2011) Outcome of combined modality treatment including neoadjuvant chemotherapy of 128 casesof locally advanced breast cancer : data from a tertiary cancer center in northern India. Indian J Cancer 48:80–85
Redkar AA, Kabre SS, Mitra I (1992) Estrogen and progesterone receptors measurement in breast cancer with enzyme-immunoassay and correlation with other prognostic factors. Indian J Med Res 96:1–8
Schott AF, Roubidoux MA, Helvie MA et al (2005) Clinical and radiologic assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 92:231–238
Schwartz GF, Birchansky CA, Komarnicky LT et al (1994) Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer 73:362–369
Sedlmayer F, Sautter-Bihl ML, Budach W, Dunst J, Fastner G, Feyer P et al (2013) DEGRO practical guidelines:radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol 189:825–833
Segal R, Dent SF, Verma S, Gerller S, Young V, Goel R et al (2006) Changing demographics of locally advanced breast cancer: data from a regional cancer centre. ASCO
Semiglazov V, Eiermann W, Zambetti M et al (2011) Surgery following neoadjuvant therapy in patients with HER2-positive locally advanced or inflammatory breast cancer participating in the NeOAdjuvant Herceptin (NOAH) study. Eur J Surg Oncol 37:856–863
Singletary SE, McNeese MD, Hortobagyi GN (1992) Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer 69:2849–2852
van der Hage JA, van de Velde CJ, Julien JP et al (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19:4224–4237
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630
Conflict of interest
All authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, U., Lakshmaiah, K.C., Govind Babu, K. et al. The actual scenario of neoadjuvant chemotherapy of breast cancer in developing country: a report of 80 cases of breast cancer from a tertiary cancer center in India. J Cancer Res Clin Oncol 140, 1777–1782 (2014). https://doi.org/10.1007/s00432-014-1724-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1724-1