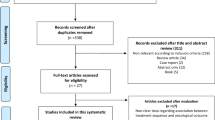
Abstract
Purpose
To assess the efficacy and toxicity of the addition of estramustine to docetaxel-based chemotherapy for the treatment of castration-resistant prostate cancer.
Methods
We systematically searched, without language restrictions, for randomized clinical trials that compared docetaxel-based chemotherapy with or without estramustine in patients with histologically proven prostate cancer. The primary end point was overall survival (OS). Secondary endpoints were prostate-specific antigen (PSA) response rate and grade 3 or 4 toxicity. Data was extracted from the studies by 2 independent reviewers. The meta-analysis was performed by Stata version 10.0 software (Stata Corporation, College Station, Texas, USA).
Results
Four randomized clinical trials (totally 400 patients) were eligible. Meta-analysis showed that there was significant improvement in PSA response rate in docetaxel-based therapy with estramustine group, compared with docetaxel-based therapy group (OR = 1.55, 95% CI = 1.10–2.18, P = 0.012). With regard to OS (HR = 0.873, 95% CI = 0.55–1.40, P = 0.572), grade3 or 4 neutropenia (OR = 1.27, 95% CI = 0.61–2.7), anemia (OR = 1.04, 95% CI = 0.07–16.3), thrombocytopenia (OR = 0.87, 95% CI = 0.13–5.7), diarrhea (OR = 2.3, 95% CI = 0.36–14.9), nausea (OR = 1.14, 95% CI = 0.16–8.35), mucositis (OR = 1.66, 95% CI = 0.50–5.52) , and vomiting (OR = 1.53, 95% CI = 0.23–10.3), and there were no significant differences between the two groups.
Conclusions
This was the first meta-analysis of docetaxel-based therapy with estramustine versus docetaxel-based chemotherapy in the treatment of castration-resistant prostate cancer. Our meta-analysis did not support the addition of estramustine to docetaxel-based chemotherapy for the treatment of castration- resistant prostate cancer, based on no gain in survival.




Similar content being viewed by others
References
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Berry DL, Moinpour CM, Jiang CS et al (2006) Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol 24:2828–2835
Caffo O, Sava T, Comploj E et al (2008) Docetaxel, with or without estramustine phosphate, as first-line chemotherapy for hormone-refractory prostate cancer: results of a multicentre, randomized phase II trial. BJU Int 102:1080–1085
Eymard JC, Priou F, Zannetti A et al (2007) Randomized phase II study of docetaxel plus estramustine and single-agent docetaxel in patients with metastatic hormone-refractory prostate cancer. Ann Oncol 18:1064–1070
Fizazi K, Le Maitre A, Hudes G et al (2007) Addition of estramustine to chemotherapy and survival of patients with castration-refractory prostate cancer: A meta-analysis of individual patient data. Lancet Oncol 8:994–1000
Gunnarsson PO, Forshell GP (1984) Clinical pharmacokinetics of estramustine phosphate. Urology 23(6 Suppl):22–27
Hahn NM, Marsh S, Fisher W et al (2006) Hoosier Oncology Group randomized phase II study of docetaxel, vinorelbine, and estramustine in combination in hormone-refractory prostate cancer with pharmacogenetic survival analysis. Clin Cancer Res 12:6094–6099
Hamilton A, Muggia F (2001) Estramustine potentiates taxane in prostate and refractory breast cancers. Oncology 15(5 Suppl 7):40–43
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics. CA Cancer J Clin 59:225–249
Machiels JP, Mazzeo F, Clausse M et al (2008) Prospective randomized study comparing docetaxel, estramustine, and prednisone with docetaxel and prednisone in metastatic hormone-refractory prostate cancer. J Clin Oncol 26:5261–5268
Oudard S, Legrier ME, Boye K et al (2003) Activity of docetaxel with or without estramustine phosphate versus mitoxantrone in androgen dependent and independent human prostate cancer xenografts. J Urol 169:1729–1734
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Perry CM, McTavish D (1995) Estramustine phosphate sodium. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in prostate cancer. Drugs Aging 7:49–74
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Williams JF, Muenchen HJ, Kamradt JM et al (2000) Treatment of androgen-independent prostate cancer using anti-microtubule agents docetaxel and estramustine in combination: an experimental study. Prostate 44:275–278
Yusuf S, Peto R, Jones DR et al (1985) Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27:335–371
Zintzaras E, Ioannidis JP (2005) Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 28:123–137
Conflict if interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Docetaxel-based Therapy with or without Estramustine as First-line Chemotherapy for Castration-resistant Prostate Cancer: A Meta-analysis of Four Randomized Controlled Trials”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, WX., Shen, Z. & Yao, Y. Docetaxel-based therapy with or without estramustine as first-line chemotherapy for castration-resistant prostate cancer: a meta-analysis of four randomized controlled trials. J Cancer Res Clin Oncol 137, 1785–1790 (2011). https://doi.org/10.1007/s00432-011-1052-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1052-7