Abstract
Purpose
Zibotentan (ZD4054) is a specific endothelin A receptor antagonist in clinical development for the treatment of hormone-resistant prostate cancer (HRPC). In a Phase II trial in patients with pain-free or mildly symptomatic metastatic HRPC, zibotentan was well tolerated with a promising signal for prolonged overall survival compared with placebo. As part of this trial, the impact of zibotentan compared with placebo on health-related quality of life (HRQoL) was assessed.
Methods
Patients were randomized to receive once-daily oral zibotentan 10 or 15 mg, or matching placebo. Patients were allocated to one of two questionnaires; the Functional Assessment of Cancer Therapy-Prostate (FACT-P) or the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), supplemented by PR25, specific for prostate cancer. Questionnaires were completed at baseline and every 4 weeks until disease progression when study treatment was discontinued.
Results
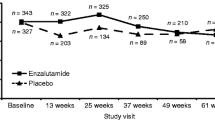
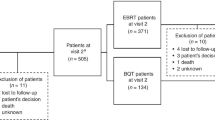
Compliance with questionnaire completion was >90% (286 of 312 patients) of the intention-to-treat population at baseline. Of baseline completers who were available for assessment (i.e., had not clinically progressed), 89% (164 of 184) and 83% (73 of 88) completed questionnaires at 12 and 24 weeks, respectively. HRQoL scores from both questionnaires were high at baseline and remained high throughout the study, with scores being similar in the zibotentan and placebo groups. However, some floor and ceiling effects were seen in the EORTC QLQ-C30 questionnaire.
Conclusions
High-baseline HRQoL scores were maintained throughout treatment with zibotentan. The FACT-P instrument was selected to further assess the impact of zibotentan on HRQoL in the Phase III clinical trial program.




Similar content being viewed by others
References
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock IF, On behalf of the TAX-327 investigators (2008) Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res 14:2763–2767
Borghede G, Sullivan M (1996) Measurement of quality of life in localized prostatic cancer patients treated with radiotherapy. Development of a prostate cancer-specific module supplementing the EORTC QLQ-C30. Qual Life Res 5:212–222
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Cella D, Nichol MB, Eton D, Nelson J, Mulani P (2008) Estimating clinically meaningful changes for the functional assessment of cancer therapy-prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 12:124–129
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ (1997) Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 50:920–928
Fayers P, Aaronson N, Bjordal K, Sullivan M (1995) EORTC QLQ-C30 scoring manual. EORTC Data Centre, Brussels
Fizazi K, Miller K (2009) Specific endothelin-A receptor antagonism for the treatment of advanced prostate cancer. BJU Int 104:1423–1425
Growcott JW (2009) Preclinical anticancer activity of the specific endothelin A receptor antagonist ZD4054. Anticancer Drugs 20:83–88
James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, Phung D, Dawson NA (2009) Safety and efficacy of the specific endothelin A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomized, Phase II trial. Eur Urol 55:1112–1123
Kemmler G, Holzner B, Kopp M, Dunser M, Margreiter R, Greil R, Sperner-Unterweger B (1999) Comparison of two quality-of-life instruments for cancer patients: the Functional Assessment Of Cancer Therapy-General and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30. J Clin Oncol 17:2932–2940
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Sullivan PW, Mulani PM, Fishman M, Sleep D (2007) Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone-refractory prostate cancer. Qual Life Res 16:571–575
Yount S, Cella D, Banik D, Ashraf T, Shevrin D (2003) Brief assessment of priority symptoms in hormone refractory prostate cancer: the FACT Advanced Prostate Symptom Index (FAPSI). Health Qual Life Outcomes 1:69
Acknowledgments
Medical writing support was provided by Matt Lewis Ph.D. of Mudskipper Bioscience on behalf of AstraZeneca. This study was sponsored by AstraZeneca (clinicaltrials.gov NCT00090363).
Conflict of interest statement
N. Dawson has received fees for travel to ASCO, GU ASCO, and SUO meetings in the United States funded by AstraZeneca; C. Battersby and M. Taboada are employees of AstraZeneca and M. Taboada also holds stocks in AstraZeneca; H. Payne, and N. James have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dawson, N., Payne, H., Battersby, C. et al. Health-related quality of life in pain-free or mildly symptomatic patients with metastatic hormone-resistant prostate cancer following treatment with the specific endothelin A receptor antagonist zibotentan (ZD4054). J Cancer Res Clin Oncol 137, 99–113 (2011). https://doi.org/10.1007/s00432-010-0864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0864-1