Abstract
Purpose
Janus tyrosine kinases (JAKs) and signal transducer and activator of transcription factors (STATs), especially STAT3, are constitutively activated in human cancers. The function of STAT3 in the pathogenesis of meningioma remains unknown. In this study, we investigated the role of JAK1/STAT3 regulating vascular endothelial growth factor (VEGF) expression in the occurrence and progression of human meningioma.
Methods
We detected the expression of JAK1, p-JAK1, STAT3, p-STAT3, and VEGF in human meningioma and normal dura tissues by RT–PCR, Western blot analysis, and immunohistochemistry.
Results
JAK1, p-JAK1, STAT3, p-STAT3, and VEGF showed high expression in grade I and grade II meningioma. The level of STAT3 activation was associated with VEGF expression; all meningioma tumors that expressed p-STAT3 also expressed VEGF. Both frequency of positivity and expression were enhanced with increasing tumor grade; high frequencies and levels were found in grade II tumors, with no expression detected in normal dura tissues (P < 0.05).
Conclusions
VEGF is directly regulated by constitutive STAT3 activity and associated with meningioma differentiation. STAT3 has an important role in the occurrence and development of human meningioma by regulating VEGF expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Constitutive activation of the Janus tyrosine kinase/signal transducer and activator of transcription (JAK/STAT) signal pathway is frequently detected in human cancers and contributes to oncogenesis. JAK1 and STAT3 are activated in response to interleukin-6 in human fibrosarcoma cells (Guschin et al. 1995), and the levels are consistently higher in low-differentiated gliomas than in high-differentiated gliomas (Cattaneo et al. 1998). Dephosphorylated JAK1 and STAT3 reduce the expression of STAT3-regulated VEGF in various human tumor cell lines (Haridas et al. 2009). As well, the JAK1/STAT3 signal pathway plays a role in cell transformation and carcinogenesis (Coppo et al. 2006; Tian et al. 1994; Tam et al. 2007). STAT3 is a member of the STAT family of STAT1-4, STAT5a, STAT5b, and STAT6, which mediates cell survival, growth, and differentiation in many cancerous cell lines and human tumors (Bowman et al. 2000; Rubin Grandis et al. 2000). Constitutively, STAT3 in tumor cells promote tumor invasion and angiogenesis by regulating the expression of target genes and modulates fundamental cellular processes, such as proliferation and differentiation.
Tumor angiogenesis is required for tumor growth and development stimulated by angiogenic inducers. VEGF, originally isolated from tumor cells, is one of the major inducers of tumor angiogenesis and regulates vascular endothelial proliferation (Ferrara et al. 2003). STAT3alpha might play a central role in activation of autocrine VEGF in gliomas (Schaefer et al. 2002). Colon cancer cells showed inhibited angiogenesis and metastasis with blocked STAT3 and VEGF expression (Kim et al. 2008). As an intracellular regulator, STAT3 is involved in tumor vascular remodeling and tumor development by regulating VEGF expression. Most brain tumors overexpress VEGF, which leads to an abnormally permeable tumor vasculature. As a solid tumor, meningioma depends on neovascularization by angiogenesis for expansion. However, the function of the JAK1/STAT3 signal pathway through mediating VEGF expression remains unknown in the pathogenesis of human meningioma.
In this study, we explored whether VEGF expression regulated by the constitutive activation of the JAK1/STAT3 signal pathway might be involved in the occurrence and development of human meningioma.
Materials and methods
Reagents
Trizol reagent was from Sigma; the first-strand cDNA synthesis kit was from Fermentas; the human primers for JAK1 (accession number NC_000001.10), STAT3 (accession number NC_000017.10), VEGFa (accession number NC_000006.11), and Beta-actin (accession number NC_000007.13) were designed with use of sequence information in GenBank and synthesized by Invitrogen; JAK1, p-JAK1 (Tyr1022/1023), p-STAT3 (Tyr705), VEGF, and Beta-actin primary antibodies were from Santa Cruz Biotechnology; STAT3 primary antibody was from Cell Signaling Technology; horseradish peroxidase-conjugated (HRP) anti-rabbit/mouse IgG secondary antibodies were from Santa Cruz Biotechnology; the DAB kit was from Zymed; the lysis buffer was from Promega; and the enhanced chemiluminescence (ECL) kit was from Amersham.
Clinical materials
We obtained 40 meningioma specimens (26 from males; donor mean age 49.0 ± 13.0 years, range 17–72 years) from the Department of Neurosurgery, the Second Hospital of Shandong University, China. None of the patients had undergone any preoperative treatments, such as chemotherapy or radiotherapy; histological differentiation was classified according to the 2000 World Health Organization classification. As much as 30 cases were grade I meningioma (well differentiated), and 10 were grade II (moderately differentiated). We obtained 6 normal dura samples from patients with cerebral trauma. The protocol was approved by the ethics committee of the Second Hospital of Shandong University, China. The patients gave their informed consent for use of samples.
One half of each sample was immediately frozen in liquid nitrogen for RT–PCR and Western blot analysis, and the other half was fixed in 15% formalin for histological typing and immunohistochemistry.
RT–PCR
Meningioma and normal samples were homogenized with 1 ml Trizol reagent; total RNA was isolated according to the manufacturer’s instructions. RNA concentration and integrity were evaluated by measuring absorbance at 260 and 280 nm, and cDNA was prepared according to the manufacturer’s instructions for the RT–PCR kit. The sequence of primers was as follows: JAK1, sense 5′-AGTGCCCTGAGCTACTTGGA-3′ and antisense 5′-AGGTCAGCCAGCTCCTTACA-3′ (371 bp); STAT3, sense 5′-AACTCTTGGGACCTGGTGTG-3′ and antisense 5′-CGGACTGGATCTGGGTCTTA-3′ (317 bp); VEGFa, sense 5′-CTGCTGTCTTGGGTGCATTG-3′ and antisense 5′-TTCACATTTGTTGTGCTGTAG-3′ (378 bp); Beta-actin, sense 5′-GTGGGGCGCCCCAGGCACCA-3′ and antisense 5′-CTCCTTAATGTCACGCACGATTTC-3′ (539 bp). A 20-μl volume reaction for PCR included 1.5 μl cDNA and 1.0 unit Taq polymerase. PCR reaction involved denaturation at 94°C for 2 min, then annealing for 32 cycles at 94°C, 55°C, and 72°C for 1 min each, with extension at 72°C for 2 min. The PCR products underwent electrophoresis on a 1% agarose gel; gene expression was normalized to that of Beta-actin by 1D Image analysis software (Eastman Kodak).
Western blot analysis
After being weighed, tumor and normal dura tissues were homogenized with liquid nitrogen, and lysis buffer was added to 200 μl; protein concentration was evaluated by measuring absorbance at 562 nm. Proteins were separated on 10% SDS–PAGE, then electroblotted on PVDF membrane; the membranes were blocked with 10% non-fat dry milk in TBS-T overnight at 4°C, then incubated with primary antibodies against JAK1, p-JAK1, STAT3, p-STAT3, VEGF, and Beta-actin (each dilution 1:1,000) for 1.5 h at room temperature. After a wash with TBS-T, membranes were incubated with HRP-conjugated secondary antibodies (dilution 1:2,000) for 1 h at room temperature. Immunoreactivity was detected by use of an ECL kit for 2–5 min according to the manufacturer’s instructions. Protein expression was normalized to that of Beta-actin by 1D Image analysis software.
Immunohistochemistry
Normal dura and tumor tissue sections (5 μm thick) were incubated at 60°C for 45 min and immediately deparaffinized in xylene (5 min × 2), then gradient ethanol (5 min each in 95%, 90%, 80%, 70%). Endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 10 min; sections were washed with phosphate buffered saline (PBS) and treated with antigen retrieval buffer in a microwave oven to unmask the antigens for 10 min. After a PBS wash, sections were blocked with diluted normal goat serum for 20 min, then primary antibodies against JAK1, p-JAK1, STAT3, p-STAT3, and VEGF (each dilution 1:100) overnight at 4°C. After a PBS wash, the appropriate secondary antibodies were added for 30 min at 37°C, then sections were washed with PBS and underwent DAB coloration for 3–5 min. The primary antibodies were substituted with PBS for negative control tissue slides for each experiment. The tissue sections were examined by two independent investigators. Protein expression was classified as follows (Preusser et al. 2005): (1) cytoplasmic staining: with 0–15% positive tumor cells was negative; <50% was low expression, ≥50% was high expression; (2) nuclear staining: with no positive tumor cells was negative; <10% was low expression, ≥10% was high expression.
Statistical analysis
SPSS v11.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Expression of genes was analyzed by independent-sample Student t test. The frequency of positivity of genes in different tissues at the mRNA and protein levels were tested by Fisher’s exact test. Spearman analysis was used to assess the correlation between gene expression and meningioma differentiation. A P < 0.05 was considered statistically significant.
Results
mRNA expression of JAK1, STAT3, and VEGFa upregulated in meningioma
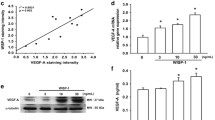
Among 30 grade I meningioma cases, JAK1 expression was detected in 11; STAT3 expression was detected in all JAK1-positive cases, and VEGFa expression was detected in all cases positive for both JAK1 and STAT3 and 1 case negative for both JAK1 and STAT3. JAK1 and STAT3 were detected at the same time in 8 out of the 10 grade II cases; VEGFa expression was found in 9 out of 10 grade II cases, including 8 cases positive for both JAK1 and STAT3. The frequencies of positivity and level of genes were high in grade I and II cases; no expression was found in normal dura tissues; high levels were found in grade II tissues (Table 1, Figs. 1, 2; P < 0.05).
RT–PCR analysis of mRNA expression of JAK1, STAT3, and VEGFa in normal dura and meningioma tissues. a mRNA levels of JAK1, STAT3, VEGFa. Beta-actin is a loading control. b Quantification of mRNA levels of JAK1, STAT3, and VEGFa normalized to that of Beta-actin. *P < 0.05 compared with normal dura tissue or grade I tumor
Plots of gene expression from RT-PCR and Western blot analysis in normal dura and meningioma tissues normalized to that of Beta-actin. Relative mRNA expression of JAK1, STAT3, and VEGFa in a normal dura tissue, b grade I tissue, and c grade II tissue. Relative protein expression of JAK1, p-JAK1, STAT3, p-STAT3, and VEGF in d normal dura tissue, e grade I tissue, and f grade II tissue
Correlation between meningioma differentiation and frequency of positivity and expression of genes by RT–PCR
The frequency of positivity and expression of genes were correlated with meningioma differentiation status (Table 1, Figs. 1, 2). The relative expression of JAK1 was strongly correlated with that of STAT3, the expression of STAT3 was also strongly correlated with that of VEGFa (Table 2).
Constitutive activation of STAT3 upregulated VEGF protein expression in meningioma
Among grade I meningioma cases, JAK1 and STAT3 expression were found in the same 11 out of 30 cases; VEGF was highly expressed in all cases expressing both JAK1 and STAT3; p-JAK1 and p-STAT3 were detected in 8 cases expressing both JAK1 and STAT3. The ratio of p-JAK1 to p-STAT3 was 72.7% (8/11). Among grade II meningioma cases, JAK1, STAT3, and VEGF were detected in the same 8 out of 10 cases; p-JAK1 and p-STAT3 expression were detected in the same 7 cases expressing JAK1, STAT3, and VEGF. The ratio of p-JAK1 to p-STAT3 was 87.5% (7/8). The relative frequency of positivity and expression of p-JAK1 and p-STAT3 were positively correlated with histological status of meningioma; high levels were found in grade II tumors (Tables 3, 4, Figs. 2, 3).
Western blot analysis of protein expression of JAK1, p-JAK1, STAT3, p-STAT3, and VEGF. a Protein level of JAK1, p-JAK1, STAT3, p-STAT3, and VEGF in normal dura and tumor tissue. Beta-actin was a loading control. b Relative protein expression of JAK1, p-JAK1, STAT3, p-STAT3, and VEGF normalized to that of Beta-actin. *P < 0.05 compared with normal dura tissues or grade I tumor
Correlation analysis of JAK1 versus STAT3, STAT3 versus VEGF, p-JAK1 versus p-STAT3, p-JAK1 versus VEGF, and p-STAT3 versus VEGF in grade I and grade II tumors showed strong correlation (Table 4). All meningioma tumors positive for p-STAT3 also showed VEGF expression. VEGF expression was also correlated with meningioma differentiation (Table 3; P < 0.05).
Constitutive activation of STAT3 upregulates VEGF protein expression associated with human meningioma differentiation
Results of frequency of positivity seen on immunohistochemistry were in agreement with those seen on Western blot analysis (Table 3). Among grade I tumors, 9 cases expressed low levels of JAK1 and STAT3 and 2 high levels; low expression was found in all p-JAK1- and p-STAT3-positive cases (8/8); 9 cases expressed a low level of VEGF and 2 a high level. Among grade II cases, both JAK1 and STAT3 were highly detected in 7, with low expression in 1 case; high levels of p-JAK1 and p-STAT3 were found in 6 cases, and low levels in 1 case; a high level of VEGF was found in all VEGF-positive cases (8/8). Relative frequency of positivity and levels of p-JAK1 and p-STAT3 were higher in grade II than in grade I tumors. Tumor tissues expressing p-STAT3 also expressed VEGF; the high levels were found in grade II tumors. Co-expression of p-STAT3 and VEGF was significantly correlated with meningioma differentiation (Tables 3, 5, Fig. 4; P < 0.05).
Immunohistochemistry of meningioma tissues (normal dura tissues not shown): a, c, e, g, i Negative control of JAK1, p-JAK1, STAT3, p-STAT3, and VEGF, respectively, in meningioma tissue. Positive protein expression of b JAK1 mainly in cytoplasm, d p-JAK1 mainly in cytoplasm, f STAT3 mainly in cytoplasm, h p-STAT3 mainly in nucleus, and j VEGF mainly in cytoplasm. Original magnification 600×
Discussion
The JAK/STAT signal pathway has been reported to be involved in the oncogenesis of human cancers. STATs are selectively activated mainly by activated JAKs (including JAK1, JAK2, JAK3, and TYK2), which leads to STAT protein activation, nuclear translocation, and regulation of target gene expression. The function of the JAK1/STAT3 signal pathway is best explained in SCID mice models: JAK1−/− and STAT3−/− mice die during embryogenesis because of impaired neurological and lymphoid development (Rodig et al. 1998; Takeda et al. 1997; Akira 1999). The JAK1/STAT3 signal pathway plays a critical role in cell transformation and carcinogenesis (Coppo et al. 2006; Tian et al. 1994; Tam et al. 2007). p-STAT3 is dysregulated in various human tumors, including head and neck squamous cell cancer (Rubin Grandis et al. 2000), leukemia (Carlesso et al. 1996), multiple myeloma (Bharti et al. 2003), and lymphomas (Weber-Nordt et al. 1996). Abnormal STAT3 activity induces permanent changes in gene expression that ultimately lead to a malignant tumor phenotype. Constitutive activation of STAT3 is associated with growth stimulation and anti-apoptotic effects in malignant disease.
In our study, we found JAK1 was significantly correlated with STAT3, and they were both over-expressed in meningioma, with the highest levels in grade II tumors; no expression was found in normal dura tissues. The expression of JAK1 and STAT3 was associated with tumor differentiation status (Table 5, Fig. 4; P < 0.05). Other studies have shown JAK1 and STAT3 markedly downregulated in the normal adult rat brain (De-Fraja et al. 2000; Gautron et al. 2006). However, JAK and STAT families of proteins are highly expressed and are important effectors in brain tumors (Magrassi et al. 1999). Our data showed varied expression of total JAK1 and STAT3 by tumor differentiation status, which suggests that the expression pattern of the JAK1/STAT3 signal pathway is associated with the development of meningioma.
Tyrosine phosphorylation is crucial for the function of STAT proteins regulated by the receptor-driven JAK catalytic activity (Lee et al. 1997; Turkson and Jove 2000). Tyrosine phosphorylation of STAT3 (p-STAT3) is a critical step for translocation to the nucleus and regulation of the expression of target genes. We found that the expression of p-JAK1 correlated with that of p-STAT3 in meningioma on Western blot analysis and immunohistochemistry; the relative expression differed by meningioma grade, with the high level in grade II tumors. The relative frequency of positivity and level of p-JAK1 and p-STAT3 were positively correlated with histological differentiation of meningioma (Tables 3, 5, Fig. 4; P < 0.05), which confirms that the activated JAK1/STAT3 signal pathway is associated with progression of human meningioma. A high level of p-STAT3 reduces patient survival in hormone-refractory prostate cancer, which suggests that activation of the JAK1/STAT3 signal pathway is involved in development of this cancer (Tam et al. 2007). So constitutive activation of JAK1/STAT3 signal pathway can be considered as an oncogenic event in the occurrence and development of tumors (Knoops et al. 2008; Zhang et al. 2000).
Protein phosphorylation has an important role in cellular processes because it regulates the functional activities of signal proteins. Activated by JAKs, phosphorylated STATs translocate from the cytoplasm to nucleus, where they regulate transcription and protein expression of target genes. Downstream targets of STAT3, such as bcl-xl, cyclin D1, and VEGF, are important in preventing apoptosis, enhancing invasion, and promoting metastasis and angiogenesis (Haridas et al. 2009; Catlett-Falcone et al. 1999; Schaefer et al. 2002; Mahboubi et al. 2001). Constitutive activation of STAT3 induces changes that lead to initiation and/or maintenance of oncogenesis. As a significant predictive factor of prognosis in patients with solid tumors, angiogenesis is essential for tumor growth, progression, and metastasis (Folkman 2001; Ohta et al. 1996). VEGF is considered as a major regulator of angiogenesis in various brain tumors (Berkman et al. 1993; Huang et al. 2005). As a solid tumor, meningioma depends on neovascularization through angiogenesis for expansion. We found high mRNA and protein levels of VEGF associated with meningioma grade. Tumor tissues expressing p-STAT3 also expressed VEGF, and p-STAT3 was significantly correlated with VEGF expression (Tables 3, 4, 5 and Figs. 2, 3, 4; P < 0.05). STAT3 can directly bind the VEGF promoter to upregulate VEGF expression in many tumors (Niu et al. 2002; Wei et al. 2003). p-STAT3 induces overexpression of VEGF, which is decreased during angiogenesis by inhibition with antagonists of VEGF–VEGFR signaling (Chen et al. 2008). So p-STAT3 is involved in angiogenesis by mediating VEGF expression, which agrees with other researches (Schaefer et al. 2002; Loeffler et al. 2005; Wang et al. 2004). The production and action of VEGF regulation by activated STAT3 is necessary in the occurrence and development of human meningioma.
Conclusion
VEGF expression is significantly correlated with STAT3 activity, and co-expression of p-STAT3 and VEGF is associated with meningioma differentiation status. STAT3 is the point of convergence for the meningioma angiogenic event as the JAK1/STAT3 signal pathway mediates VEGF expression. So STAT3 has an important role in the occurrence and development of human meningioma.
Abbreviations
- JAK1:
-
Janus tyrosine kinase 1
- p-JAK1:
-
Phosphorylated Janus tyrosine kinase 1
- p-STAT3:
-
Phosphorylated signal transducer and activator of transcription 3
- STAT3:
-
Signal transducer and activator of transcription 3
- VEGF:
-
Vascular endothelial growth factor
References
Akira S (1999) Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 17:138–146
Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, Robertson JT, Ali IU, Oldfield EH (1993) Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest 91:153–159
Bharti AC, Donato N, Aggarwal BB (2003) Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol 171:3863–3871
Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19:2474–2488
Carlesso N, Frank DA, Griffin JD (1996) Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med 183:811–820
Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, Dalton WS, Jove R (1999) Constitutive activation of STAT3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10:105–115
Cattaneo E, Magrassi L, De-Fraja C, Conti L, Di Gennaro I, Butti G, Govoni S (1998) Variations in the levels of the JAK/STAT and ShcA proteins in human brain tumors. Anticancer Res 18:2381–2387
Chen SH, Murphy DA, Lassoued W, Thurston G, Feldman MD, Lee WM (2008) Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer Biol Ther 7:1994–2003
Coppo P, Flamant S, De Mas V, Jarrier P, Guillier M, Bonnet ML, Lacout C, Guilhot F, Vainchenker W, Turhan AG (2006) BCR-ABL activates STAT3 via JAK and MEK pathways in human cells. Br J Haematol 134:171–179
De-Fraja C, Conti L, Govoni S, Battaini F, Cattaneo E (2000) STAT signalling in the mature and aging brain. Int J Dev Neurosci 18:439–446
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Folkman J (2001) Angiogenesis-dependent diseases. Semin Oncol 28:536–542
Gautron L, De Smedt-Peyrusse V, Layé S (2006) Characterization of STAT3-expressing cells in the postnatal rat brain. Brain Res 1098:26–32
Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark GR (1995) A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J 14:1421–1429
Haridas V, Nishimura G, Xu ZX, Connolly F, Hanausek M, Walaszek Z, Zoltaszek R, Gutterman JU (2009) Avicin D: a protein reactive plant isoprenoid dephosphorylates STAT3 by regulating both kinase and phosphatase activities. PLoS One 4:e5578
Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R (2005) Expression of VEGF and its receptors in different brain tumors. Neurol Res 27:371–377
Kim ES, Hong SY, Lee HK, Kim SW, An MJ, Kim TI, Lee KR, Kim WH, Cheon JH (2008) Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol Rep 20:1321–1327
Knoops L, Hornakova T, Royer Y, Constantinescu SN, Renauld JC (2008) JAK kinases overexpression promotes in vitro cell transformation. Oncogene 27:1511–1519
Lee CK, Bluyssen HA, Levy DE (1997) Regulation of interferon-alpha responsiveness by the duration of Janus kinase activity. J Biol Chem 272:21872–21877
Loeffler S, Fayard B, Weis J, Weissenberger J (2005) Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer 115:202–213
Magrassi L, De-Fraja C, Conti L, Butti G, Infuso L, Govoni S, Cattaneo E (1999) Expression of the JAK and STAT superfamilies in human meningiomas. J Neurosurg 91:440–446
Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y, Carroll JM, Elias JA, Altieri DC, Pober JS (2001) Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab Invest 81:327–334
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H (2002) Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21:2000–2008
Ohta Y, Endo Y, Tanaka M, Shimizu J, Oda M, Hayashi Y, Watanabe Y, Sasaki T (1996) Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res 2:1411–1416
Preusser M, Birner P, Ambros IM, Ambros PF, Budka H, Harris AL, Hainfellner JA (2005) DEC1 expression in 1p-aberrant oligodendroglial neoplasms. Histol Histopathol 20:1173–1176
Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM Jr, Schreiber RD (1998) Disruption of the JAK1 gene demonstrates obligatory and nonredundant roles of the JAKs in cytokine-induced biologic responses. Cell 93:373–383
Rubin Grandis J, Zeng Q, Drenning SD (2000) Epidermal growth factor receptor-mediated STAT3 signaling blocks apoptosis in head and neck cancer. Laryngoscope 110:868–874
Schaefer LK, Ren Z, Fuller GN, Schaefer TS (2002) Constitutive activation of STAT3alpha in brain tumors: localization to tumor endothelial cells and activation by the endothelial tyrosine kinase receptor (VEGFR-2). Oncogene 21:2058–2065
Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S (1997) Targeted disruption of the mouse STAT3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 94:3801–3804
Tam L, McGlynn LM, Traynor P, Mukherjee R, Bartlett JM, Edwards J (2007) Expression levels of the JAK/STAT pathway in the transition from hormone-sensitive to hormone-refractory prostate cancer. Br J Cancer 97:378–383
Tian SS, Lamb P, Seidel HM, Stein RB, Rosen J (1994) Rapid activation of the STAT3 transcription factor by granulocyte colony-stimulating factor. Blood 84:1760–1764
Turkson J, Jove R (2000) STAT proteins: novel molecular targets for cancer drug discovery. Oncogene 27:6613–6626
Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN (2004) Analysis of the activation status of Akt, NFkappaB, and STAT3 in human diffuse gliomas. Lab Invest 84:941–951
Weber-Nordt RM, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart V, Mertelsmann R, Finke J (1996) Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood 88:809–816
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K (2003) STAT3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22:319–329
Zhang Y, Turkson J, Carter-Su C, Smithgall T, Levitzki A, Kraker A, Krolewski JJ, Medveczky P, Jove R (2000) Activation of STAT3 in v-Src-transformed fibroblasts requires cooperation of JAK1 kinase activity. J Biol Chem 275:24935–24944
Acknowledgments
We gratefully acknowledge the financial support of the Science Department of Shandong Province, China (2007BS03063).
Conflict of interest statement
The authors declare that no any financial and personal relationships with other people or organisations that can inappropriately influence the work, they have full control of all primary data and agree to allow the journal to review if requested.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. X. Zhang and X. Zhao contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zhang, M.X., Zhao, X., Wang, Z.G. et al. Constitutive activation of signal transducer and activator of transcription 3 regulates expression of vascular endothelial growth factor in human meningioma differentiation. J Cancer Res Clin Oncol 136, 981–988 (2010). https://doi.org/10.1007/s00432-009-0743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0743-9