Abstract
Purpose
Predictive factors for response to docetaxel in human breast cancers have yet to be identified. The aim of the present study was to investigate the relationship of various clinicopathological and biological parameters with pathological response to docetaxel in the neoadjuvant setting.
Methods
The study population comprised 78 patients with primary breast cancers who were treated with docetaxel [60 mg/m2; four (median) cycles, range 3–6; q3w] as neoadjuvant therapy and subsequently treated with mastectomy or breast conserving surgery. Tumor samples obtained before chemotherapy were subjected to histological examination and immunohistochemistry of HER-2 and Ki-67.
Results
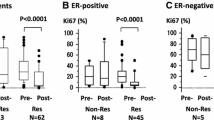
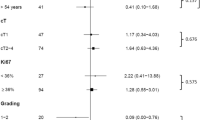
The pathological complete response (pCR) rate was significantly (P = 0.04) higher for tumors with low nuclear grade (NG-I or -II) (21%) than for tumors with high NG (NG-III) (5%). The pCR rate (20%) of small (≤5 cm) tumors was marginally significantly (P = 0.05) higher than that of large (>5 cm) tumors (5%). Combined analysis of NG and tumor size showed that low-NG small tumors have a higher response rate (30%) than high-NG small tumors (11%; P = 0.13), low-NG large tumors (11%; P = 0.15), and high-NG large tumors (0%; P = 0.009). No statistically significant association was observed between pCR rate and menopausal status, lymph node status, ER, PR, HER-2, or Ki-67.
Conclusions
Low nuclear grade, but not cell proliferation determined by Ki-67, is associated with a good pathological response to docetaxel. Combination of low nuclear grade and small tumor size may be useful for the selection of breast tumors with a high pCR rate (30%).


Similar content being viewed by others
References
Amat S, Bougnoux P, Penault-Llorca F, Fetissof F, Cure H, Kwiatkowski F, Achard JL, Body G, Dauplat J, Chollet P (2003) Neoadjuvant docetaxel for operable breast cancer induces a high pathological response and breast-conservation rate. Br J Cancer 88:1339–1345
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N, National Surgical Adjuvant Breast, Bowel Project Protocol B-27 (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21:4165–4174
Bottini A, Berruti A, Bersiga A, Brizzi MP, Brunelli A, Gorzegno G, DiMarco B, Aguggini S, Bolsi G, Cirillo F, Filippini L, Betri E, Bertoli G, Alquati P, Dogliotti L (2000) p53 but not bcl-2 immunostaining is predictive of poor clinical complete response to primary chemotherapy in breast cancer patients. Clin Cancer Res 6:2751–2758
Burcombe RJ, Makris A, Richman PI, Daley FM, Noble S, Pittam M, Wright D, Allen SA, Dove J, Wilson GD (2005) Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer 92:147–155
Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Elledge R, Mohsin S, Osborne CK, Chamness GC, Allred DC, O’Connell P (2003) Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 362:362–369
Di Leo A, Chan S, Paesmans M, Friedrichs K, Pinter T, Cocquyt V, Murray E, Bodrogi I, Walpole E, Lesperance B, Korec S, Crown J, Simmonds P, Von Minckwitz G, Leroy JY, Durbecq V, Isola J, Aapro M, Piccart MJ, Larsimont D (2004) HER-2/neu as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Breast Cancer Res Treat 86:197–206
Diaz JF, Andreu JM (1993) Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry 32:2747–2755
Estevez LG, Cuevas JM, Anton A, Florian J, Lopez-Vega JM, Velasco A, Lobo F, Herrero A, Fortes J (2003) Weekly docetaxel as neoadjuvant chemotherapy for stage II and III breast cancer: efficacy and correlation with biological markers in a phase II, multicenter study. Clin Cancer Res 9:686–692
Fernandez-Sanchez M, Gamboa-Dominguez A, Uribe N, Garcia-Ulloa AC, Flores-Estrada D, Candelaria M, Arrieta (2006) Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med Oncol 23:171–183
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Fraizer GC, Diaz MF, Lee IL, Grossman HB, Sen S (2004) Aurora-A/STK15/BTAK enhances chromosomal instability in bladder cancer cells. Int J Oncol 25:1631–1639
Garcia P, Braguer D, Carles G, el Khyari S, Barra Y, de Ines C, Barasoain I, Briand C (1994) Comparative effects of taxol and Taxotere on two different human carcinoma cell lines. Cancer Chemother Pharmacol 34:335–343
Hasegawa S, Miyoshi Y, Egawa C, Ishitobi M, Taguchi T, Tamaki Y, Monden M, Noguchi S (2003) Prediction of response to docetaxel by quantitative analysis of class I and III beta-tubulin isotype mRNA expression in human breast cancers. Clin Cancer Res 9:2992–2997
Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, Marumoto T, Saya H, Horii A (2005) RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 65:2899–2905
Hu W, Kavanagh JJ, Deaver M, Johnston DA, Freedman RS, Verschraegen CF, Sen S (2005) Frequent overexpression of STK15/Aurora-A/BTAK and chromosomal instability in tumorigenic cell cultures derived from human ovarian cancer. Oncol Res 15:49–57
Inaji H, Kobayashi K (2005) The Journal of the Japanese Breast Cancer Society: Ggeneral rules for clinical and pathological recording of breast cancer. Breast Cancer Suppl 12:S9
Iwao-Koizumi K, Matoba R, Ueno N, Kim SJ, Ando A, Miyoshi Y, Maeda E, Noguchi S, Kato K (2005) Prediction of docetaxel response in human breast cancer by gene expression profiling. J Clin Oncol 23:422–431
Jeng YM, Peng SY, Lin CY, Hsu HC (2004) Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res 10:2065–2071
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17:460–469
Miyoshi Y, Ando A, Takamura Y, Taguchi T, Tamaki Y, Noguchi S (2002) Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int J Cancer 97:129–132
Molino A, Micciolo R, Turazza M, Bonetti F, Piubello Q, Bonetti A, Nortilli R, Pelosi G, Cetto GL (1997) Ki-67 immunostaining in 322 primary breast cancers: associations with clinical and pathological variables and prognosis. Int J Cancer 74:433–437
Murata K, Sato T, Kanamaru R (1994) Effect of a new anticancer drug, docetaxel (RP56976), on human leukemia cell lines. Gan To Kagaku Ryoho 21:307–313
Penault-Llorca F, Cayre A, Bouchet Mishellany F, Amat S, Feillel V, Le Bouedec G, Ferriere JP, De Latour M, Chollet P (2003) Induction chemotherapy for breast carcinoma: predictive markers and relation with outcome. Int J Oncol 22:1319–1325
Prisack HB, Karreman C, Modlich O, Audretsch W, Danae M, Rezai M, Bojar H (2005) Predictive biological markers for response of invasive breast cancer to anthracycline/ cyclophosphamide-based primary (radio-)chemotherapy. Anticancer Res 25:4615–4621
Ravdin PM, Valero V (1995) Review of docetaxel (Taxotere), a highly active new agent for the treatment of metastatic breast cancer. Semin Oncol Suppl 4:17–21
Ravdin PM, Burris HA 3IIIrd, Cook G, Eisenberg P, Kane M, Bierman WA, Mortimer J, Genevois E, Bellet RE (1995) Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol 13: 2879–2885
Seidman AD, Hudis C, Crown JPA (1993) Phase II evaluation of Taxotere (RP56976 NSC 628503) as initial chemotherapy for metastatic breast cancer. Proc Am Soc Clin Oncol 12:63
Takamura Y, Kobayashi H, Taguchi T, Motomura K, Inaji H, Noguchi S (2002) Prediction of chemotherapeutic response by collagen gel droplet embedded culture-drug sensitivity test in human breast cancers. Int J Cancer 98:450–455
Tatsuka M, Sato S, Kitajima S, Suto S, Kawai H, Miyauchi M, Ogawa I, Maeda M, Ota T, Takata T (2005) Overexpression of Aurora-A potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene 24:1122–1127
ten Bokkel Huinink WW, Prove AM, Piccart M, Steward W, Tursz T, Wanders J, Franklin H, Clavel M, Verweij J, Alakl M, Bayssas M, Kaye SB (1994) A phase II trial with docetaxel (Taxotere) in second line treatment with chemotherapy for advanced breast cancer. A study of the ORTC Early Clinical Trials Group. Ann Oncol 5:527–532
Tong T, Zhong Y, Kong J, Dong L, Song Y, Fu M, Liu Z, Wang M, Guo L, Lu S, Wu M, Zhan Q (2004) Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res 10:7304–7310 Erratum in (2005) Clin Cancer Res 11:4635
Tsuda H, Sasano H, Akiyama F, Kurosumi M, Hasegawa T, Osamura RY, Sakamoto G (2002) Evaluation of interobserver agreement in scoring immunohistochemical results of HER-2/neu (c-erbB-2) expression detected by HercepTest, Nichirei polyclonal antibody, CB11 and TAB250 in breast carcinoma. Pathol Int 52:126–134
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19:4224–4237
Vincent-Salomon A, Rousseau A, Jouve M, Beuzeboc P, Sigal-Zafrani B, Freneaux P, Rosty C, Nos C, Campana F, Klijanienko J, Al Ghuzlan A, Sastre-Garau X, Breast Cancer Study Group (2004) Proliferation markers predictive of the pathological response and disease outcome of patients with breast carcinomas treated by anthracycline-based preoperative chemotherapy. Eur J Cancer 40:1502–1508
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Breast Cancer Society, for Cancer Research from the Ministry of Health, Labour and Welfare of Japan, and for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Miyoshi, Y., Kurosumi, M., Kurebayashi, J. et al. Low nuclear grade but not cell proliferation predictive of pathological complete response to docetaxel in human breast cancers. J Cancer Res Clin Oncol 134, 561–567 (2008). https://doi.org/10.1007/s00432-007-0319-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-007-0319-5