Abstract
Purpose
PTEN is a tumor suppressor gene that inhibits cell proliferation by regulating intracellular signaling pathways, and this activity can be abolished by mutations of the PTEN gene. This study was designed to examine the correlation of PTEN expression with the expression of cell cycle regulators and with clinicopathological parameters in endometrioid adenocarcinoma of the uterine corpus.
Methods
Tissue samples of 117 endometrioid adenocarcinomas in addition to those of 19 normal endometria and 20 endometrial hyperplasias were used for the study. Immunohistochemical staining for PTEN protein was performed with the labeled streptavidin-biotin method on formalin-fixed and paraffin-embedded tissue samples. PTEN expression was represented as the staining score.
Results
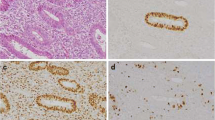
Immunohistochemistry showed that the nuclei of cells were positive for PTEN. The PTEN staining score of normal endometrium was significantly higher in the proliferative phase than in the secretory phase. The scores of various endometrial hyperplasias were not significantly different from each other, regardless of the type of hyperplasia. The PTEN staining scores of endometrioid adenocarcinomas were 7.6±5.2 in G1, 9.6±5.2 in G2, and 11.9±3.7 in G3, and increased significantly as the histological grade increased. PTEN staining score was not significantly correlated with clinicopathological parameters such as FIGO stage, myometrial invasion, lymph-vascular space invasion (LVSI), lymph node metastasis or group, but was significantly correlated with labeling indices (LIs) of cell cycle regulators such as Ki-67, cdk2, cyclin A, cyclin D1, cyclin E, p27, and p53. The PTEN staining score of p53-wild cases was significantly lower than that of p53-mutant ones, but there was no significant difference of the score in cases with different PTEN gene status. PTEN expression was significantly lower in cases with both high levels of estrogen receptor and progesterone receptor.
Conclusion
PTEN protein expression was decreased in well-differentiated and less growth-aggressive endometrial carcinoma with wild-type p53 gene and high levels of ER and PR. This suggests that disturbed PTEN expression occurs in an early phase of the tumorigenesis of well-differentiated endometrial carcinoma.



Similar content being viewed by others
References
Bussaglia E, DEL Rio E, Matias-Guiu X, Prat J (2000) PTEN mutations in endometrial carcinomas: a molecular and clinicopathologic analysis of 38 cases. Hum Pathol 31:312–317
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H (2001) Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor α. J Biol Chem 276:9817–9824
de la Cuesta RS, Eichhorn JH, Rice LW, Fuller Jr AF, Nikrui N, Goff BA (1996) Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol 60:238–244
Ellenson LH (2000) The molecular biology of endometrial tumorigenesis: does it have a message? Int J Gynecol Pathol 19:310–313
Fujimoto J, Hirose R, Sakaguchi H, Tamaya T (1998) Estrogen dependency in uterine endometrial cancers. Oncology 55:53–59
Fujisawa T, Watanabe J, Akaboshi M, Ohno E, Kuramoto H (2001) Immunohistochemical study on VEGF expression in endometrial carcinoma – comparison with p53 expression, angiogenesis, and tumor histologic grade. J Cancer Res Clin Oncol 127:668–674
Gimm O, Perren A, Weng L-P, Marsh DJ, Yeh JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, Mutter GL, Robinson BG, Komminoth P, Dralle H, Eng C (2000) Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 156:1693–1700
Girl D, Ittmann M (1999) Inactivation of the PTEN tumor suppressor gene is associated with increased angiogenesis in clinically localized prostate carcinoma. Hum Pathol 30:419–424
Gu J, Tamura M, Yamada KM (1998) Tumor supressor PTEN inhibits integrin-and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol 143:1375–1383
Hata H, Hamano M, Watanabe J, Kuramoto H (1998) Role of estrogen and estrogen-related growth factor in the mechanism of hormone dependency of endometrial carcinoma cells. Oncology 55:35–44
Hinoda Y, Idogawa M, Imai K (1998) Involvement of protein tyrosine phosphatases in cancer development. Protein Nucleic Acid Enzyme 43:1186–1192
Kato N, Watanabe J, Jobo T, Nishimura Y, Fujisawa T, Kamata Y, Kuramoto H, (2003) Immunohistochemical expression of cyclin E in endometrial adenocarcinoma (endometrioid type) and its clinicopathological significance. J Cancer Res Clin Oncol 129:222–226
Kato S, Kitamoto T, Masuhiro Y, Yanagisawa J (1998) Molecular mechanism of a cross-talk between estrogen and growth-factor signaling pathways. Oncology 55:5–10
Kohler MF, Berchuck A, Davidoff AM, Humphrey PA, Dodge RK, Iglehart JD, Soper JT, Clarke-Pearson DL, Bast RC Jr, Marks J (1992) Overexpression and mutation of p53 in enometrial carcinoma. Cancer Res 52:1622–1627
Kurose K, Bando K, Fukino K, Sugisaki Y, Araki T, Emi M, (1998) Somatic mutations of the PTEN/MMAC1 gene in fifteen Japanese endometrial cancers: evidence for in activation of both alleles. Jpn J Cancer Res 89:842–848
Kyushima N, Watanabe J, Hata H, Jobo T, Kameya T, Kuramoto H (2002) Expression of cyclin A in endometrial adenocarcinoma and its correlation with proliferative activity and clinicopathological variables. J Cancer Res Clin Oncol 128:307–312
Levine RL, Cargile CB, Blazes MS, Rees Bv, Kurman RJ, Ellenson LH (1998) PTEN mutations and microsatellite instability in complex atypical hyperplasia, a Precursor lesion to uterine endometorioid carcinoma. Cancer Res 58:3254–3258
Maehama T, Dixon JE (2000) Function of PTEN as a phospholipid phosphatase. Cell Technol 19:751–753
Maxwell GL, Risinger JI, Gumbs C, Shaw H, Bentley RC, Barrett JC, Berchuck A, Futreal PA (1998) Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res 58:2500–2503
Mochizuki Y (1999) Tumor suppressor gene PTEN/MMAC1 is lipid phosphatase. Exp Med 17:1195–1199
Mutter GL (2000a) Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol 19:301–309
Mutter GL, Lin M-C, Fitzgerald JT, Kum JB, Baak JPA, Lee JA, Weng L-P, Eng C (2000b) Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 92:924–931
Mutter GL, Lin M-C, Fitzgerald JT, Kum JB, Eng C (2000c) Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab 85:2334–2338
Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG (1998) Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res 58:2095–2097
Ohkawara S, Jobo T, Sato R, Kuramoto H (2000) Comparison of endometrial carcinoma coexisting with and without endometrial hyperplasia. Eur J Gynaec Oncol 6:573–577
Ohtani K, Sakamoto H, Satoh K (1999) Molecular pathogenesis of endometrial hyperplasia and adenocarcinoma. Nihon Univ J Med 41:181–193
Orita M, Suzuki Y, Sekiya T, Hayashi K (1989) Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics 5:874–879
Parsons R (1998) Phosphatases and tumorigenesis. Curr Opin Oncol 10:88–91
Perren A, Weng L-P, Boag AH, Ziebold U, Thakore K, Dahia PLM, Komminoth P, Lees JA, Mulligan LM, Mutter GL, Eng C (1999) Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 155:1253–1260
Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, Hess KR, Myers JN, Hong Y-K, Yung WKA, Steck PA (1999) Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res 59:1820–1824
Sherman ME (2000) Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol 13:295–308
Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677
Steck PA, Pershouse MA, Jasser SA, Yung WKA, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DHF, Tavtigian SV (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet 15:356–362
Tamura M, Gu J, Yamada KM (1998a) Tumor suppressor PTEN: a negative regulator of cell adhesions via integrins. Exp Med 16:2211–2213
Tamura M, Gu J, Matsumoto K, Aota S, Persons R, Yamada KM (1998b) Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280:1614–1617
Tamura M, Gu J, Takino T, Yamada KM, (1999) Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130cas. Cancer Res 59:442–449
Uchida T, Wada C, Shitara T, Egawa S, Koshiba K (1993) Infrequent involvement of p53 gene mutations in the tumorigenesis of Japanese prostate cancer. Br J Cancer 68:751–755
Watanabe J, Sato H, Kanai T, Kamata, Y, Jobo T, Hata H, Fujisawa T, Ohno E, Kuramoto H (2002) Paradoxical expression of cell cycle inhibitor p27 in endometrioid adenocarcinoma of the uterine corpus — correlation with proliferation and clinicopathological parameters. Br J Cancer 87:81–85
Watanabe J, Kamata Y, Kanai T, Seo N, Fujisawa T, Nishimura Y, Hamano M, Jobo T, Kuramoto H (2003) Expression of cell cycle regulators in endometrial adenocarcinoma. Kuramoto H, Nishida M (eds) Cell and molecular biology of endometrial carcinoma. Springer, Tokyo, pp 93–106
Weng LP, Brown JL, Eng C (2001) PTEN coordinates G1 arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet 10:599–604
Acknowledgments
This work was supported by grants-in-aid for the Project Research of Graduate School of Medical Sciences, Kitasato University (Grants 2005 and 4007) and for Scientific Research from the Ministry of Education, Culture, Sports, Sciences, and Technology (Grants 12670176 and 12671627), Japan
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, F., Watanabe, J., Hata, H. et al. PTEN immunohistochemical expression is suppressed in G1 endometrioid adenocarcinoma of the uterine corpus. J Cancer Res Clin Oncol 130, 161–168 (2004). https://doi.org/10.1007/s00432-003-0517-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0517-8