Abstract
Purpose
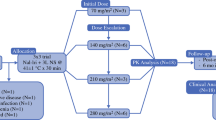
To perform a phase I study of intraperitoneal cis-bis-neodecanoato (trans-R, R-1, 2-diaminocyclohexane)-platinum II entrapped in multilamellar vesicles (L-NDDP) for peritoneal carcinomatosis or sarcomatosis.
Methods
Eligible patients had normal renal, hematologic, and liver functions. Laparoscopy was performed on the first two courses for evaluation, adhesiolysis, and chemotherapy administration. Afterwards, chemotherapy was administered through a peritoneal catheter. Up to six courses were allowed. Peritoneal imaging with technetium-labeled sulfur colloid was used to determine adequate distribution prior to each course. Volunteering patients underwent pharmacokinetics studies during the second course.
Results
Fifteen of 16 registered patients, seven women and eight men (median age 53 years (range 26–76) and median performance status of 1) were assessable. Diagnoses were: malignant mesothelioma (six patients), signet ring cell (three), colon adenocarcinoma, pseudomyxoma peritonei, gastrointestinal stromal tumor (two each), and ovarian carcinoma (one). Median number of courses was two (range, one to six) Dose-limiting toxicity symptoms were fatigue and abdominal pain. Hematologic toxicities were minimal. Peri-operative complications included one colonic perforation requiring primary closure, a peritoneal catheter malfunction, a port site hematoma, and an ascites leak requiring re-suture. Five patients survived at least 3 years. Pharmacokinetics studies indicated a rapid but low absorption of drug into the systemic circulation, with a prolonged retention of platinum in the plasma compartment. Peritoneal L-NDDP exposure was 17 to 49-times greater than in the plasma compartment.
Conclusions
Peritoneal cavity exposure to L-NDDP is prolonged, and systemic absorption is limited, yielding a high peritoneal/plasmatic ratio. The recommended dose for phase II studies is 400 mg/m2 every 28 days.


Similar content being viewed by others
References
Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Isawa E, Sumida M, Ohkubo H (1997) Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 79:884
Griffiths CT, Fuller AF (1978) Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am 58:131
Jacquet P, Stephens AD, Averbach AM, Chang D, Ettinghausen SE, Dalton RR, Steves MA, Sugarbaker PH (1996) Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 77:2622
Sugarbaker PH, Zhu BW, Sese GB, Shmookler B (1993) Peritoneal carcinomatosis from appendiceal cancer: results in 69 patients treated by cytoreductive surgery and intraperitoneal chemotherapy. Dis Colon Rectum 36:323
Sugarbaker PH, Jablonski KA (1995) Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy (comments). Ann Surg 221:124
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19:1001
Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer (comments). N Engl J Med 335:1950
Armstrong DK, Bundy BN, Baergen R, Lele SB, Copeland LJ, Walker J, Burger R (2002) Randomized phase III study of intravenous (IV) paclitaxel and cisplatin versus IV paclitaxel, intraperitoneal (IP) cisplatin and IP paclitaxel in optimal stage III epithelial ovarian cancer (OC): a Gynecologic Oncology Group trial (GOG 172). A803
Fujimura T, Yonemura Y, Fushida S, Urade M, Takegawa S, Kamata T, Sugiyama K, Hasegawa H, Katayama K, Miwa K (1990) Continuous hyperthermic peritoneal perfusion for the treatment of peritoneal dissemination in gastric cancers and subsequent second-look operation. Cancer 65:65
Esquivel J, Vidal-Jove J, Steves MA, Sugarbaker PH (1993) Morbidity and mortality of cytoreductive surgery and intraperitoneal chemotherapy (comments). Surgery 113:631
Perez-Soler R, Shin DM, Siddik ZH, Murphy WK, Huber M, Lee SJ, Khokhar AR, Hong WK (1997) Phase I clinical and pharmacological study of liposome-entrapped NDDP administered intrapleurally in patients with malignant pleural effusions. Clin Cancer Res 3:373
Siddik ZH, Boxall FE, Harrap KR (1987) Flameless atomic absorption spectrophotometric determination of platinum in tissues solubilized in hyamine hydroxide. Anal Biochem 163:21
Perez-Soler R, Lopez-Berestein G, Lautersztain J, al-Baker S, Francis K, Macias-Kiger D, Raber MN, Khokhar AR (1990) Phase I clinical and pharmacological study of liposome-entrapped cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum(ii). Cancer Res 50:4254
Alberts DS, Markman M, Armstrong D, Rothenberg ML, Muggia F, Howell SB (2002) Intraperitoneal therapy for stage III ovarian cancer: a therapy whose time has come! J Clin Oncol 20:3944
Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T (1988) Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 61:232
Hamazoe R, Maeta M, Kaibara N (1994) Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 73:2048
Speyer JL (1985) The rationale behind intraperitoneal chemotherapy in gastrointestinal malignancies. Semin Oncol 12:23
Markman M (1994) Intraperitoneal cisplatin and carboplatin in the management of ovarian cancer. Semin Oncol 21:17
Lind M, Murphy D, Sharma H, Tinker N, Smith A, McAuliffe C, Crowther D (1991) Comparative intraperitoneal pharmacokinetics of three platinum analogues. Cancer Chemother Pharmacol 28:315
Elferink F, Vijgh WVD, Klein I, Huinink WTB, Dubbelman R, McVie J (1988) Pharmacokinetics of carboplatin after intraperitoneal administration. Cancer Chemother Pharmacol 21:57
Marchettini P, Stuart OA, Mohamed F, Yoo D, Sugarbaker PH (2002) Docetaxel: pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemother Pharmacol 49:499
Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, Stoter G, Sparreboom A (2002) Influence of Cremophor El on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res 8:1237
Francis P, Rowinsky E, Schneider J, Hakes T, Hoskins W, Markman M (1995) Phase I feasibility and pharmacologic study of weekly intraperitoneal paclitaxel: a Gynecologic Oncology Group pilot study. J Clin Oncol 13:2961
Perez-Soler R, Francis K, al-Baker S, Pilkiewicz F, Khokhar AR (1994) Preparation and characterization of liposomes containing a lipophilic cisplatin derivative for clinical use. J Microencapsul 11:41
Khokhar AR, al-Baker S, Brown T, Perez-Soler R (1991) Chemical and biological studies on a series of lipid-soluble (trans-(R,R)- and -(S,S)-1,2-diaminocyclohexane)platinum(II) complexes incorporated in liposomes. J Med Chem 34:325
Perez-Soler R, Yang LY, Drewinko B, Lauterzstain J, Khokhar AR (1988) Increased cytotoxicity and reversal of resistance to cis-diamminedichloro-platinum(ii) with entrapment of cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexaneplatinum (ii) in multilamellar lipid vesicles. Cancer Res 48:4509
Perez-Soler R, Lautersztain J, Stephens LC, Wright K, Khokhar AR (1989) Preclinical toxicity and pharmacology of liposome-entrapped cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum(II). Cancer Chemother Pharmacol 24:1
Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y, Sasaki T (2001) Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 48:1776
Elias DM, Ouellet JF (2001) Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin North Am 10:915
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verschraegen, C.F., Kumagai, S., Davidson, R. et al. Phase I clinical and pharmacological study of intraperitoneal cis-bis-neodecanoato(trans-R, R-1, 2-diaminocyclohexane)-platinum II entrapped in multilamellar liposome vesicles. J Cancer Res Clin Oncol 129, 549–555 (2003). https://doi.org/10.1007/s00432-003-0481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0481-3