Abstract
Purpose. The aim of the present study was to determine the turnover (4-hydroxylation and N-dechloroethylation) of ifosfamide in a total of 25 human liver microsomal preparations in which the codetermination of keto- and carboxyifosfamide as well as the calculation of free and protein-bound acrolein was carried out for the first time.
Methods. The 4-hydroxylation of ifosfamide was estimated by using acrolein (free and protein-bound) and a newly developed procedure involving the codetermination of keto- and carboxyifosfamide (LC/MS). The ifosfamide N-dechloroethylation was determined as the sum of 2- and 3-dechloroethylifosfamide (LC/MS).
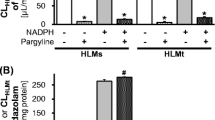
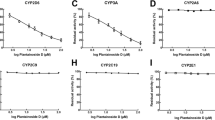
Results. Using the usual estimation of liberated free acrolein in 25 human liver microsomal preparations, the 4-hydroxylation of ifosfamide amounted to 0.28±0.16 nmol/min · nmolP450. However, after calculating the 4-hydroxylation as the sum of free and protein-bound acrolein and keto- and carboxyifosfamide, a ninefold higher activity (2.40±0.73 nmol/min · nmolP450) was found. The percentage of the inactive metabolites keto- (25/25) and carboxyifosfamide (5/25) in the 4-hydroxylation amounted to only 0.79–5.25% (mean 2.90%). The ifosfamide N-dechloroethylation (mean 0.21±0.11 nmol/min · nmolP450) determined as the sum of 2- and 3-dechloroethylifosfamide was estimated as 8.3±4.3% of the total ifosfamide turnover. The application of the relative substrate-activity factor (RSF)-approach and the calculation of the contribution of various isoforms in the ifosfamide 4-hydroxylation yielded the following results: CYP 3A4: 58±31%, CYP 2A6: 25±15%, and CYP 2C9: 5±2% of the total measured 4-hydroxylation. A correlation between 4-hydroxylation and the N-dechloroethylation rates of ifosfamide and the activities of isoenzymes indicates the involvement of both CYP 3A4 (P=0.026) and CYP 2C9 (P=0.012) in the 4-hydroxylation reaction and of CYP 3A4 (P<0.01) in the N-dechloroethylation reaction.
Conclusions. The estimation of protein-bound acrolein should be included in the calculation of the ifosfamide 4-hydroxylation besides liberated free acrolein. Because of the small amounts of the inactive metabolites keto- and carboxyifosfamide, the exclusive determination of acrolein only (free and protein-bound) seems to suffice for the calculation of total ifosfamide hydroxylation. Using this method the hepatic in vitro turnover of ifosfamide was estimated as 92% for 4-hydroxylation (CYP 3A4 and CYP 2A6 mediated) and 8% for N-dechloroethylation (CYP 3A4 mediated), and in this way, a relative overestimation of the N-dechloroethylation of ifosfamide on the whole metabolism is avoided.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Preiss, R., Schmidt, R., Baumann, F. et al. Measurement of 4-hydroxylation of ifosfamide in human liver microsomes using the estimation of free and protein-bound acrolein and codetermination of keto- and carboxyifosfamide. J Cancer Res Clin Oncol 128, 385–392 (2002). https://doi.org/10.1007/s00432-002-0335-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00432-002-0335-4