Abstract
Skeletal muscle atrophy is known to be a marker for nutritional deficiency. The diaphragm is both a skeletal muscle and a respiratory muscle. There is not enough data in the literature about the change in diaphragm thickness (DT) in children with malnutrition. We think that malnutrition may have negative effects on diaphragm thickness. Therefore, in this study, we aimed to compare the diaphragm thicknesses of pediatric patients with primary malnutrition and a healthy control group. The DT of pediatric patients diagnosed with primary malnutrition by a pediatric gastroenterologist was prospectively evaluated by a radiology specialist by ultrasonography (USG). The obtained data were statistically compared with the data of the healthy control group. There was no statistically significant difference between the groups in terms of age and gender (p = 0.244, p = 0.494). We found that right and left diaphragm thicknesses were significantly thinner in the malnourished group than in the healthy control group (p = 0.001, p = 0.009, respectively). We found that right and left diaphragm thicknesses were thinner in those with moderate and severe malnutrition compared to the normal group (p < 0.001, p = 0.003, respectively). We found a significant weak positive correlation between weight and height Z score and right and left diaphragm thickness (respectively, r: 0.297, p < 0.001; r: 0.301, p < 0.001).
Conclusion: Malnutrition is a disease that affects all systems. Our study shows that the DT is thinner in patients with malnutrition.
What is Known: •Malnutrition causes skeletal muscle atrophy. | |
What is New: •Diaphragm muscle thickness decreases in malnutrition. •There is a significant positive correlation between diaphragm muscle thickness and height, weight and BMI z scores. |
Similar content being viewed by others
Introduction
Malnutrition is a systemic disease that occurs as a result of insufficient intake or absorption of macro- or micronutrients necessary for the body to perform organ functions. Malnutrition can cause skeletal muscle atrophy. Skeletal muscle atrophy is an important marker that affects the length of stay in hospital, intensive care unit, and mechanical ventilation [1].
The diaphragm is also a skeletal muscle closely related to respiratory function. More than half of the tidal volume in breathing is generated by diaphragmatic muscle contractions. In the presence of severe disease, their contraction is reduced [2, 3]. Therefore, diaphragmatic atrophy has a significant impact on the prognosis of patients with lower respiratory tract infections [4]. Dynamic monitoring of skeletal muscle (diaphragm) in malnutrition allows real-time assessment of nutritional status and can guide nutritional therapy [5]. We think that atrophy may develop in the diaphragm muscle in malnutrition. For this purpose, we aimed to measure the diaphragm thickness (DT) of malnourished patients with ultrasonography (USG), which is an inexpensive and non-invasive method that allows easy measurement of DT and can dynamically evaluate diaphragm function [6,7,8] and movement, and compare it with healthy control group.
Materials and methods
Between 2021 and 2022, patients aged 1 month to 18 years who were diagnosed with primary malnutrition in the Pediatric Gastroenterology outpatient clinic of Kahramanmaraş Sütçü İmam University Hospital were evaluated by a radiology specialist in terms of diaphragm thickness. The data obtained were statistically compared with the data of the healthy control group from the same age group.
Ethics committee approval was obtained for this study from the local tertiary university hospital Faculty of Medicine Local Ethics Committee (decision no: 2021/14: 04). The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from the patients’ parents before the study.
Study group
Primary malnutrition
Primary malnutrition occurs as a result of insufficient and unbalanced intake of nutrients necessary for growth and development due to environmental and economic reasons. Secondary malnutrition is due to an underlying disease [9].
Evaluation of nutritional status
Measurements in patients included in the study are the following:
-
Height was measured to the nearest millimeter using a calibrated vertical portable stadium meter without socks and shoes.
-
Weight was measured using a digital electronic scale adjusted to the nearest decimal fraction of a kilogram while wearing light clothing.
Weight, height, and body mass index (BMI) Z score were calculated according to age and gender using World Health Organization (WHO) data. Patients with a Z score below − 1 in any of the body weight, height, and BMI parameters were considered malnourished.
The severity of malnutrition was classified as follows [10,11,12,13]:
-
Normal: weight-for-age Z score > − 1 SDS
-
Mild underweight: weight-for-age Z score − 2 ≤ to ≤ − 1
-
Moderate underweight: weight-for-age Z score − 3 ≤ to < − 2
-
Severe underweight: weight-for-age Z score < − 3
-
Normal: height-for-age Z score > − 1 SDS
-
Mild stunting: height-for-age Z score − 2 ≤ to ≤ − 1
-
Moderate stunting: height-for-age Z score − 3 ≤ to < − 2
-
Severe stunting: height-for-age Z score < − 3
-
Normal: BMI-for-age Z score > − 1 SDS
-
Mild wasting: BMI-for-age Z score −2 ≤ to ≤ − 1
-
Moderate wasting: BMI-for-age Z score − 3 ≤ to < − 2
-
Severe wasting: BMI-for-age Z score < − 3.
The following criteria were used for the diagnosis of obesity [14]:
-
Obesity: up to 5 years: BMI Z score ≥ (+ 3)
-
> 5 years old; those with a BMI Z score (+ 2) were diagnosed with obesity.
-
Patients with obesity criteria were not included in the study.
Healthy control group
Patients in the same age group without malnutrition (with a weight Z score < 2 and > − 1) or without known systemic chronic disease were included. Obese patients were not included.
Exclusion criteria
Patients with secondary malnutrition, obesity, diabetes, congenital heart disease, heart failure, kidney failure, chronic liver disease, asthma, cystic fibrosis, or chest deformity (pectus excavatum, pectus carinatum) were excluded from the study.
Ultrasound evaluation
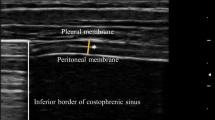
Ultrasonographic evaluations were performed by a single radiologist with 10 years of experience in thoracic ultrasonography. A LOGIQ E9 XDclear 2.0 ultrasound machine (GE Healthcare, 2017, USA) and linear transducer (6–15 MHz xd, Matrix clear) were used for this. The auditor was blinded to the study groups. The probe was placed over the anterior axillary line and the chest wall. When the probe was moved caudally, the diaphragm was seen as a hypoechoic structure between the two hyperechoic lines. Measurements were taken from the most caudal part of the diaphragm. The diaphragm muscle between the upper pleural line and the lower peritoneal line was measured. Pleural and peritoneal lines were not included in the measurement (Fig. 1). The thickness of the diaphragm was measured with electronic calipers. Measurements were taken in the supine position, at the end of expiration, from the right and left sides. These measurements were made in millimeters (mm). This technique is reliable and easy to apply [15,16,17].
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 software. The normality of the distribution of the data was tested using visual (histogram and probability charts) and analytical methods (Kolmogorov–Smirnov and Shapiro–Wilk tests). Descriptive analyses were presented as percentile, mean, and standard deviation. Normally distributed numerical data were compared using the independent samples t test and non-normally distributed numerical data were compared using the Mann–Whitney U test. The chi-square test was used to compare the frequency rates of categorical variables. The one-way ANOVA test was used to detect the differences between the means of more than two independent groups and to show between which groups this difference is and to emphasize its importance. ROC curve analysis was performed to determine the best cut-off points for diaphragm thickness measurements to detect underfeeding. Logistic regression analysis was performed to evaluate whether the diaphragm thickness cut-off point determined by ROC curve analysis poses a risk for malnutrition. Correlation analysis was performed to determine whether there is a linear relationship between two numerical variables and, if so, the direction and severity of this relationship. If these numerical data were normally distributed, Pearson correlation was preferred; otherwise, Spearman rank correlation was preferred. A P value of < 0.05 was considered statistically significant.
Results
The mean age of the patients was 8.03 ± 5.63 (0.25–17.9) years. A total of 125 patients with primary malnutrition and 55 healthy control subjects were included in the study. There were 75 (60%) girls in the malnutrition patient group and 30 (54.5%) girls in the healthy control group. The mean age was found as 8.767 ± 4.644 years in the patients in the malnutrition group and 7.703 ± 6.004 years in the healthy control group. There was no statistically significant difference between the groups in terms of age and gender (p = 0.244, p = 0.494) (Table 1).
We found that the right and left DT were significantly lower in the malnutrition group compared to those in the healthy control group (p < 0.001, p = 0.003 respectively) (Table 1).
There was no significant difference in terms of right and left diaphragm thicknesses according to gender (p = 0.699, p = 0.263; respectively) (Table 2).
When the right and left diaphragm thicknesses were evaluated according to the weight Z scores of the patients, we found that right and left diaphragm thicknesses were significantly thinner in moderately and severely malnutrition patients than in normal-weight patients (p = < 0.001, p = 0.009, respectively) (Table 3).
When the DT was evaluated according to the height Z score of the patients, we found the right DT to be significantly thinner in patients with severe stunting than those with normal height and mild stunting (p = 0.007). In addition, we found the left DT to be thinner in those with moderate stunting than in those with normal and mild stunting (p = 0.014) (Table 3).
The degree of malnutrition was determined according to the BMI Z score of the patients and the diaphragm thicknesses were compared. According to this, right diaphragm thicknesses of patients with moderate and severe malnutrition were found to be significantly lower than of the healthy control group (p = 0.016). Although the left DT was thinner in patients with moderate and severe malnutrition, there was no statistically significant difference between the groups (p = 0.114) (Table 3).
There was a significant positive correlation between right and left DT and weight and height Z scores (weight r: 0.297, p = < 0.001; r: 0.258, p = < 0.001 and height r: 0.301, p = < 0.001; r: 0.291, p = < 0.001, respectively).
But regarding their correlation with BMI Z scores, there was a weak significant correlation between the right DT and BMI Z scores, while no statistically significant correlation between the left DT and BMI Z scores (r: 0.159, p = 0.034; r: 0.138, p = 0.066, respectively) (Table 4).
There was a significant positive correlation between age and the right and left DT (r: 0.426, p < 0.001; r: 0.337, p < 0.001, respectively) (Table 5). But according to different age groups, the DT was significantly thinner in the malnutrition group than in the control group in infants and those above 8 years only (Table 6).
We performed ROC curve analysis to determine the best cut-off point for diaphragm thickness to determine malnutrition for 0–1 years, 1–4 years, 4–8 years, and > 8 years. We determined that it would detect malnutrition with 70% sensitivity, 66.4% specificity, and p = 0.030 significance in patients with right diaphragm thickness ≤ 1.45 mm only in the > 8 age group. We could not find a statistically significant cut-off point in other age groups (Table 7). We performed a risk analysis with logistic regression analysis according to this cut-off point determined in patients > 8 years old. We showed that the risk of malnutrition increased 2.86 times in patients aged > 8 years with a right diaphragm thickness of ≤ 1.45 mm (p = 0.002) (Table 8).
Discussion
There were various studies in the literature investigating the relationship between diaphragm thickness and some systemic diseases. For example, Şahin et al. reported that diaphragm thickness in multiple sclerosis patients was similar to the healthy group [15]. On the other hand, studies were reporting a higher diaphragm thickness due to possible pseudohypertrophy in myopathies and a positive correlation between lung functions and diaphragm thickness and extension [15, 16, 18,19,20,21].
Traditional nutritional monitoring parameters such as prealbumin, albumin, transferrin, mid-arm circumference, and skinfold thickness are sensitive to inflammation and edema and may give false results. They have low predictive value for the assessment of nutritional status in severely ill patients [22]. CT is considered the gold standard for the assessment of skeletal muscle amount, and the applicability of the psoas muscle index in the assessment of nutritional status has been demonstrated [23, 24]. The diaphragm is both respiratory and skeletal muscle. It may be more useful than other skeletal muscles in evaluating nutritional status and determining prognosis in critically ill patients. Ultrasonography seems to be more advantageous in the diagnosis of skeletal muscle atrophy due to the radiation risk and cost of CT. Ultrasonography is a valid and reliable imaging method in the evaluation of DT and gives an idea about the diaphragm morphology. Compared to other imaging methods, USG has many advantages such as ease of application, low cost, dynamic imaging, bedside evaluation, high resolution, non-invasive, and no ionizing radiation [16, 25,26,27]. USG also allows us to observe sudden changes in diaphragm thickness and to evaluate extubation time in mechanically ventilated patients [28].
There is no consensus in the literature about which moments of the respiratory cycle are better for diaphragm thickness measurement by ultrasonography [17, 29]. It has been reported in the literature that diaphragm thickness may differ in supine position, sitting position, inspiration, and expiration [16]. Therefore, in our study, we preferred to measure in the supine position and at the end of expiration in all cases so that we could evaluate our measurements objectively. We could find a limited number of articles in the literature related to nutritional status and diaphragm thickness. You et al. [30] reported that the diaphragm thickness obtained with CT could reflect the patient’s whole body muscle mass and was significantly associated with nutritional status and hospital stay. In addition, it was stated that changes in diaphragm thickness occur dynamically during the course of the disease in COVID-19 patients and may be a dynamic nutritional status assessment tool. In their study, they showed that there was a negative correlation between nutritional risk and diaphragm thickness in hospitalized COVID-19 patients, and the diaphragm thickness decreased within 2 weeks due to nutritional deficiency. Unlike You et al. [30], we preferred ultrasonography in our study. Our study group consisted of pediatric patients differently. In our study, we found the diaphragm thickness to be thinner in patients with malnutrition than in the healthy control group. We found a significant positive correlation between weight, height Z scores, and diaphragm thickness. Our findings support the work of You et al. [30].
Rehan et al. [31] reported that there was a positive relationship between diaphragm thickness and body size in their study in healthy term infants. van Doorn et al. [32] reported that there was a weak positive correlation between BMI and diaphragm thickness, and that the diaphragm thickness in men was thicker than in women of the same age group. In our study, we found a significant positive correlation between weight, height, and BMI Z scores and right diaphragm thickness. In addition, there was no significant difference between the groups in terms of diaphragm thickness according to gender. van Doorn et al. [32] found end-expiratory diaphragm thicknesses of 1.4 mm and 1.3 mm, and end-inspiratory diaphragm thicknesses of 3.2 mm and 3.3 mm, respectively, in children aged 0–9 years and 10–19 years. They also reported that the measurements at the end of inspiration and expiration may be different. We also measured the diaphragm thicknesses at the end of expiration in our study. The healthy group data in our study supports the end-expiratory diaphragm measurements of van Doorn et al. [32]. Differently, we found that the diaphragm thickness was thinner in the malnutrition group than in the healthy control group of the same age. In addition, we found that the diaphragm thickness became thinner in pediatric patients with primary malnutrition, unlike healthy children. We found a moderate positive correlation between diaphragm thickness and age in the malnutrition patient group (Table 5). This is due to skeletal muscle atrophy that develops as a result of nutritional deficiency. In addition, the insufficient muscle reserve in young children compared to older children may explain the thin diaphragm thicknesses in malnutrition patients. These results are an indication that malnutrition may adversely affect the prognosis, especially in infants.
Duyndam et al. [33] reported normal values of diaphragmatic thickness and diaphragm thickening fraction using ultrasonography in 137 healthy children aged 0–8 years. They found that the diaphragm thicknesses of babies up to 1 year old were thicker than those of children aged 2–8 years. They also reported that the diaphragm thickness may decrease as the body surface area increases. Our study includes pediatric patients between the ages of 0 and 18 and consists of a malnourished and healthy control group. Consistent with the literature, we found the right diaphragm thickness to be significantly thicker between 0 and 1 years of age in the healthy group than between 1.1 and 8 years of age (p = 0.041). However, although the diaphragm thickness appears physiologically thin between 1.1 and 8 years of age, it seems to thicken again over the age of 8 years (Table 6). This may be due to the gradual decrease in rapid growth, hypertrophy secondary to use, and anabolic hormones exposed during adolescence.
We determined the most accurate right diaphragm thickness as ≤ 1.45 mm to detect malnutrition in children over 8 years of age. We showed that the risk of malnutrition increased 2.86 times in these children. We could not detect a significant cut-off point in the left diaphragm thickness (Tables 7 and 8). We think that this difference between the right and left diaphragms is due to the easier evaluation of the right diaphragm. Because the right diaphragm is viewed through the liver window, and the left diaphragm is viewed through the spleen window. The right diaphragm is easier to evaluate than the left diaphragm because of the limited left splenic window and intervention from the stomach [34]. For this reason, we recommend using the right diaphragm thickness for measurements.
The small sample size and the inability to measure control diaphragm thicknesses after malnutrition treatment are the limitations of our study.
The strengths of our study are that it is a rare prospective study evaluating diaphragm thickness in pediatric patients with primary malnutrition and a healthy group and comparing it with anthropometric measurements.
As a result, malnutrition is a disease that affects all systems. Our study shows that the diaphragm thickness is thinner in malnourished patients compared to that in the healthy control group, and the diaphragm becomes thinner as the age decreases in malnourished patients. This finding suggests that in conditions such as severe lower respiratory tract infection and heavy exercise, critical effort capacity may decrease and adversely affect the prognosis. However, these findings need to be supported by more extensive randomized controlled trials.
References
Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, Beishuizen A (2014) Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care (London, England) 18(2):R12. https://doi.org/10.1186/cc13189
Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM (2012) Diaphragm muscle thinning in patients who are mechanically ventilated. Chest 142(6):1455–1460. https://doi.org/10.1378/chest.11-1638
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, Bolz SS et al (2015) Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med 192(9):1080–1088. https://doi.org/10.1164/rccm.201503-0620OC
Sklar MC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, Rittayamai N, Harhay MO, Reid WD, Tomlinson G, Rozenberg D (2020) Association of low baseline diaphragm muscle mass with prolonged mechanical ventilation and mortality among critically ill adults. JAMA Net Open 3(2):e1921520. https://doi.org/10.1001/jamanetworkopen.2019.21520
Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Velloso C et al (2013) Acute skeletal muscle wasting in critical illness. JAMA 310(15):1591–1600. https://doi.org/10.1001/jama.2013.278481
Dhungana A, Khilnani G, Hadda V, Guleria R (2017) Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Crit Care Med 6(4):185–189. https://doi.org/10.5492/wjccm.v6.i4.185
Beck J, Weinberg J, Hamnegård CH, Spahija J, Olofson J, Grimby G, Sinderby C (2006) Diaphragmatic function in advanced Duchenne muscular dystrophy. Neuromuscul Disord 16(3):161–167. https://doi.org/10.1016/j.nmd.2006.01.003
Boon AJ, Sekiguchi H, Harper CJ, Strommen JA, Ghahfarokhi LS, Watson JC, Sorenson EJ (2014) Sensitivity and specificity of diagnostic ultrasound in the diagnosis of phrenic neuropathy. Neurology 83(14):1264–1270. https://doi.org/10.1212/WNL.0000000000000841
Shahrin L, Chisti MJ, Ahmed T (2015) 3.1 Primary and secondary malnutrition. World Rev Nutr Diet 113:139–146. https://doi.org/10.1159/000367880
Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A (2017) Severe childhood malnutrition. Nat Rev Dis Primers 3:17067. https://doi.org/10.1038/nrdp.2017.67
Bouma S (2017) Diagnosing pediatric malnutrition. Nutr Clin Pract 32(1):52–67. https://doi.org/10.1177/0884533616671861
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85(9):660–667. https://doi.org/10.2471/blt.07.043497
Mehta NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney L, Monczka JL, Plogsted SW, Schwenk WF, American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors (2013) Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN. J Parenter Enteral Nutr 37(4):460–481. https://doi.org/10.1177/0148607113479972.
de Onis M, Lobstein T (2010) Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes 5(6):458–460. https://doi.org/10.3109/17477161003615583
Şahin H, Doğan A, Ekiz T (2019) Ultrasonographic evaluation of the diaphragm thickness in patients with multiple sclerosis. Mult Scler Relat Disord 36:101369. https://doi.org/10.1016/j.msard.2019.08.011.
Fayssoil A, Behin A, Ogna A, Mompoint D, Amthor H, Clair B, Laforet P, Mansart A, Prigent H, Orlikowski D, Stojkovic T, Vinit S, Carlier R, Eymard B, Lofaso F, Annane D (2018) Diaphragm: pathophysiology and ultrasound imaging in neuromuscular disorders. J Neuromuscul Dis 5(1):1–10. https://doi.org/10.3233/JND-170276
Haaksma ME, Smit JM, Boussuges A, Demoule A, Dres M, Ferrari G, Formenti P, Goligher EC, Heunks L, Lim EHT, Mokkink LB, Soilemezi E, Shi Z, Umbrello M, Vetrugno L, Vivier E, Xu L, Zambon M, Tuinman PR (2022) EXpert consensus on diaphragm ultrasonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Critical Care (London, England) 26(1):99. https://doi.org/10.1186/s13054-022-03975-5
Gottesman E, McCool FD (1997) Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 155(5):1570–1574. https://doi.org/10.1164/ajrccm.155.5.9154859
Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T (2016) Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve 53(4):522–527. https://doi.org/10.1002/mus.24902
De Bruin PF, Ueki J, Bush A, Khan Y, Watson A, Pride NB (1997) Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax 52(5):472–475. https://doi.org/10.1136/thx.52.5.472
Jung JH, Kim NS (2017) The correlation between diaphragm thickness, diaphragmatic excursion, and pulmonary function in patients with chronic stroke. J Phys Ther Sci 29(12):2176–2179. https://doi.org/10.1589/jpts.29.2176
Ferrie S, Tsang E (2018) Monitoring nutrition in critical illness: what can we use? Nutr Clin Pract 33(1):133–146. https://doi.org/10.1177/0884533617706312
Bahat G, Turkmen BO, Aliyev S, Catikkas NM, Bakir B, Karan MA (2021) Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nut (Edinburgh, Scotland) 40(6):4360–4365. https://doi.org/10.1016/j.clnu.2021.01.010
Wang NC, Zhang P, Tapper EB, Saini S, Wang SC, Su GL (2020) Automated measurements of muscle mass using deep learning can predict clinical outcomes in patients with liver disease. Am J Gastroenterol Suppl 115(8):1210–1216. https://doi.org/10.14309/ajg.0000000000000662
Khurana J, Gartner SC, Naik L, Tsui BCH (2018) Ultrasound identification of diaphragm by novices using ABCDE technique. Reg Anesth Pain Med 43(2):161–165. https://doi.org/10.1097/AAP.0000000000000718
Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, Brochard LJ, Bolz SS, Rubenfeld GD, Kavanagh BP, Ferguson ND (2015) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intens Care Med 41(4):642–649. https://doi.org/10.1007/s00134-015-3687-3
Özçakar L, Kara M, Chang KV, Çarl AB, Akkaya N, Tok F, Chen WS, Wang TG, Tekin L, Ulaşl AM, Chen CP, Çapkn E, De Muynck M (2015) Nineteen reasons why physiatrists should do musculoskeletal ultrasound: EURO-MUSCULUS/USPRM recommendations. Am J Phys Med Rehabil 94(6):e45–e49. https://doi.org/10.1097/PHM.0000000000000223
Dionísio MT, Rebelo A, Pinto C, Carvalho L, Neves JF (2019) Avaliação Ecográfica da Disfunção Diafragmática Induzida pelo Ventilador em Idade Pediátrica [Ultrasound assessment of ventilator-induced diaphragmatic dysfunction in paediatrics]. Acta Med 32(7–8):520–528. https://doi.org/10.20344/amp.10830
Schmid B, Boussuges A (2019) Assessment of diaphragmatic function by ultrasonography: a device to record breathing signals on an ultrasound machine. Biomed J Sci Tech Res 15:11032–11036
You Y, Chen M, Chen X, Yu W (2022) Diaphragm thickness on computed tomography for nutritional assessment and hospital stay prediction in critical COVID-19. Asia Pac J Clin Nutr 31(1):33–40. https://doi.org/10.6133/apjcn.202203_31(1).0004
Rehan VK, McCool FD (2003) Diaphragm dimensions of the healthy term infant. Acta Paediatr (Oslo, Norway: 1992) 92(9):1062–1067
van Doorn JLM, Wijntjes J, Saris CGJ, Ottenheijm CAC, van Alfen N, Doorduin J (2022) Association of diaphragm thickness and echogenicity with age, sex, and body mass index in healthy subjects. Muscle Nerve 66(2):197–202. https://doi.org/10.1002/mus.27639
Duyndam A, Smit J, Heunks L, Molinger J, IJland M, van Rosmalen J, van Dijk M, Tibboel D, Ista E (2023) Reference values of diaphragmatic dimensions in healthy children aged 0–8 years. Eur J Pediatr. https://doi.org/10.1007/s00431-023-04920-6
Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A (1994) Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax 49(9):885–889. https://doi.org/10.1136/thx.49.9.885
Author information
Authors and Affiliations
Contributions
Şükrü Güngör: investigation, methodology, project administration, roles/writing - original draft, resources. Adil Doğan: data curation, formal analysis, methodology, project administration, writing - review and editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Güngör, Ş., Doğan, A. Diaphragm thickness by ultrasound in pediatric patients with primary malnutrition. Eur J Pediatr 182, 3347–3354 (2023). https://doi.org/10.1007/s00431-023-05024-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05024-x