Abstract
This multi-center point prevalence study evaluated children who were diagnosed as having coronavirus disease 2019 (COVID-19). On February 2nd, 2022, inpatients and outpatients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were included in the study from 12 cities and 24 centers in Turkey. Of 8605 patients on February 2nd, 2022, in participating centers, 706 (8.2%) had COVID-19. The median age of the 706 patients was 92.50 months, 53.4% were female, and 76.7% were inpatients. The three most common symptoms of the patients with COVID-19 were fever (56.6%), cough (41.3%), and fatigue (27.5%). The three most common underlying chronic diseases (UCDs) were asthma (3.4%), neurologic disorders (3.3%), and obesity (2.6%). The SARS-CoV-2-related pneumoniae rate was 10.7%. The COVID-19 vaccination rate was 12.5% in all patients. Among patients aged over 12 years with access to the vaccine given by the Republic of Turkey Ministry of Health, the vaccination rate was 38.7%. Patients with UCDs presented with dyspnea and pneumoniae more frequently than those without UCDs (p < 0.001 for both). The rates of fever, diarrhea, and pneumoniae were higher in patients without COVID-19 vaccinations (p = 0.001, p = 0.012, and p = 0.027).
Conclusion: To lessen the effects of the disease, all eligible children should receive the COVID-19 vaccine. The illness may specifically endanger children with UCDs.
What is Known: • Children with COVID-19 mainly present with fever and cough, as in adults. • COVID-19 may specifically threaten children with underlying chronic diseases. | |
What is New: • Children with obesity have a higher vaccination rate against COVID-19 than children without obesity. • Among unvaccinated children, fever and pneumoniae might be seen at a higher ratio than among vaccinated children. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been continuing for nearly 3 years and the Omicron variant and its lineages are still circulating [1, 2]. Multiple approaches have been used to prevent its spread and protect vulnerable people. At first, it was considered that children might be one of the risky groups. However, from the beginning, children have overcome the disease easily, mostly with mild effects [3, 4]. In time, COVID-19 vaccines were developed, and a less severe course of COVID-19 began to be seen [5]. The Centers for Disease Control and Prevention (CDC) now recommend that children be vaccinated from age 6 months [6]. However, vaccine accessibility has been given only to children > 12 years in Turkey since the summer of 2021 [7].
This study evaluated multi-center point prevalence data of children who were diagnosed as having COVID-19 to determine a cross-sectional view of pediatric cases. In this way, we wanted to show a snapshot of the single-day status of pediatric COVID-19 in Turkey. The comorbidities and vaccination status of the patients with COVID-19 were also examined.
Materials and methods
Study design and participants
This study was designed as a multi-center, point prevalence study. Pediatric patients who were diagnosed as having COVID-19 on February 2nd, 2022, using polymerase chain reaction (PCR) from nasopharyngeal swab samples were included in the study. Twenty-four centers located in 12 cities and five regions in Turkey participated in the study. The patients were chosen from the hospital’s emergency departments and outpatient and inpatient clinics where suspected COVID-19 cases were evaluated.
Data collection
The total number of admissions and the number of patients who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on February 2nd, 2022, were recorded. The demographic, clinical, and laboratory characteristics of the patients infected with SARS-CoV-2 were obtained. Demographic characteristics included age (months), sex, and the vaccination status of the patients and their parents. Clinical characteristics included COVID-19 symptoms, the patients’ underlying chronic diseases (UCDs), and their requirements in follow-up, such as oxygen or mechanical ventilation, antibiotic use, and pediatric intensive care unit (PICU) need. Laboratory characteristics including complete blood count values, C-reactive protein (CRP), ferritin, and D-dimer were recorded.
Statistical analyses
The SPSS 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) statistics package program was used in data analysis. Data are presented as the number and percentage of observations (n, %), mean ± standard deviation, and range. The results of homogeneity (Levene’s test) and normality (Kolmogorov–Smirnov test) were used to decide the statistical methods for comparing the study groups. Among normally distributed groups with homogeneous variances, dependent groups were compared using Student’s t-test. According to the test results, parametric test assumptions were unavailable for some variables; therefore, the independent groups were compared using the Mann–Whitney U test. For more than two group comparisons of independent variables, the Kruskal–Wallis test was used as a non-parametric test. Categorical data were analyzed using Fisher’s exact test and the chi-square test. Statistical significance was defined as p values less than 0.05.
Patients’ medical reports
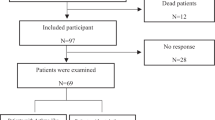
Overall, 8605 patients were evaluated on February 2nd, 2022, 706 (8.2%) of whom had SARS-CoV-2 PCR positivity. The median age of the 706 patients with COVID-19 was 92.50 [interquartile range (IQR): 24–163.25] months. Among the 706 patients, 127 (18%) were aged under 12 months, 150 (21.2%) were aged 1–5 years, 197 (27.9%) were aged 5–12 years, and 232 (32.9%) were aged 12–18 years. Three hundred seventy-seven (53.4%) were girls, and 329 (46.6%) were boys. The three most common symptoms of the patients with COVID-19 were fever (56.6%), cough (41.3%), and fatigue (27.5%) (Fig. 1). One hundred thirty-eight patients (19.7%) had at least one UCD. The most common underlying diseases were asthma (3.4%), neurologic disorders (3.3%), obesity (2.6%), congenital heart diseases (1.7%), hypertension (1.3%), and diabetes mellitus (0.6%), all of which are shown in Table 1 along with the demographic, laboratory, and clinical characteristics of the patients.
Of the 706 patients, 76.7% (n = 542) were evaluated as outpatients. Among the inpatients (n = 164, 23.2%), 8.6% needed PICU admission. The clinical differences between the inpatients and outpatients are shown in Table 2. The overall PICU admission rate was 1.8%. Seventy-five (10.7%) patients had SARS-CoV-2-related pneumoniae, all of whom were inpatients. None of the patients received antiviral agents in the treatment of COVID-19. Nearly one in four patients (24.9%) was given antibiotics due to secondary bacterial infections, and in clinical courses that could not be differentiated from bacterial pneumoniae. The COVID-19 vaccination rate was 12.5% in all patients. Among patients aged > 12 years who had access to the vaccine given by the Republic of Turkey Ministry of Health, the vaccination rate was 38.7%. The vaccination statuses of the patients and their parents are detailed in Table 3.
Results
Comparisons of the clinical features and the most widely seen UCDs are shown in Table 4. Unremarkable results were not included in the table. Patients with UCDs had a higher prevalence of dyspnea and pneumoniae than those without UCD (p < 0.001 for both). They were also evaluated in the PICU more commonly and needed high-flow oxygen and mechanical ventilation more frequently than those without UCDs (p < 0.001 for both).
When vaccination status was evaluated between patients with or without chronic diseases, patients with obesity were detected as having higher vaccination rates compared with patients without obesity [(n = 7/8) 87.5% vs. (n = 67/181) 37%, respectively; p = 0.006]. Patients with general chronic conditions, diabetes, hypertension, asthma, neurologic disorders, and congenital heart diseases showed no statistical differences in vaccination rates (p = 0.787, p = 0.521, p = 0.301, p = 0.381, p = 0.157, and p = 0.999, respectively).
In the laboratory analyses, the patients’ median white blood cell count was lower in patients with UCDs (8500 vs. 7450 × 103/µL, respectively; p = 0.043). The median absolute lymphocyte count of the patients was lower in patients with UCDs (3075 vs. 2200 × 103/µL, respectively; p < 0.001). The median platelet counts of the patients were lower in patients with UCDs (312 vs. 255 × 103/µL, respectively; p = 0.004). The median CRP levels of the patients were higher in patients with UCDs (6.3 vs. 9 mg/L, respectively; p = 0.018). Absolute neutrophil count, D-dimer, and ferritin levels did not differ between the groups with and without UCDs (p = 0.822, p = 0.120, and p = 0.328, respectively).
The clinical features examined among vaccinated and unvaccinated children are categorized in Table 5. Despite being statistically nonsignificant (p = 0.214), the percentage of unvaccinated children with dyspnea was higher than among children without dyspnea (92.7% vs. 86.9%). On the other hand, unvaccinated children presented with fever and pneumoniae at a higher rate than vaccinated children (91.4% vs. 81.9% for fever, 95.7% vs. 86.4% for pneumoniae) (p = 0.001 and p = 0.027, respectively). Headache, sore throat, fatigue, and myalgia were seen in higher ratios in vaccinated than unvaccinated patients (< 0.001, < 0.001, < 0.001, 0.010, respectively). Other symptoms depicted in Fig. 1 were not statistically significant between unvaccinated and vaccinated patients. Only significant analyses were added to the table. Cough and dyspnea were included in the table despite their nonsignificance because they were among the most prominent symptoms of COVID-19. There were no differences in steroid and low-molecular-weight-heparin use among vaccinated and unvaccinated patients. However, antimicrobial therapy was preferred mostly in unvaccinated patients with COVID-19 (p < 0.001). There was no difference between vaccinated and unvaccinated patients in terms of laboratory analyses (p > 0.05 for all) except for absolute lymphocyte count, which was lower in vaccinated patients (p = 0.024).
Discussion
Point prevalence studies are cross-sectional studies that provide a topographic view that helps to obtain important information regarding a situation. This research is the first point prevalence study conducted in Turkey investigating pediatric patients with COVID-19. Our COVID-19 positivity rate was 8.2% among children in the participating centers on February 2nd, 2022. The World Health Organization stated Turkey’s positivity rate at the time as 10.01% across the entire population [8]. The hospitalization rate was 23.2% in our study. It was 62% in Götzinger et al.’s multi-center study with 582 pediatric patients [9]. Their high hospital admission rate could be attributable to the wild virus, which was more aggressive than the recent Omicron variant and its sub-lineages, because the study was conducted in 2020.
In our study, the median age of the patients was 92.50 months, which was similar to Dong et al.’s study [10]. Female patients were predominant in contrast to the literature [11, 12]. From the beginning of the pandemic, the two most common symptoms were fever and cough, as shown in most studies [13,14,15], which was compatible with our research with rates of 56.6% for fever and 41.3% for cough.
Regarding respiratory support requirements and PICU admissions, our patients were in small proportions (6.4% and 1.8%, respectively). In Götzinger et al.’s study, the PICU admission rate was 8%, which was considerably higher than in our research [9]. Christophers et al. found the pneumoniae rate as 13.8%, and antiviral treatment, including lopinavir/ritonavir, oseltamivir, and ribavirin, was given to 23.6% of patients [14]. The pneumoniae rate was 10.7% in our study and more than a quarter of hospitalized patients were found to have pneumonia, suggesting that pneumonia may be a contributing cause of hospitalization. However, we observed no antiviral use for pneumoniae related to COVID-19 on the study day in our multi-center study. Studies investigating the benefits of antiviral use in children with COVID-19 are scarce [16]. Remdesivir can be used in severe cases or mild-to-moderate cases that are predicted to worsen [17]. The reason we did not encounter the use of remdesivir may be due to the inability to access the agent. In COVID-19 treatment, steroids should also be considered, particularly for those with distinct inflammatory responses [18]. Hence, steroids were used in certain patients in our research on a case-by-case basis. Nearly one-quarter of the patients were given antibiotics. The attitude to initiate antimicrobials during COVID-19 infections indicates that there are still concerns about secondary bacterial pneumonia. However, it has been reported to be rare in the literature [19,20,21].
The rate of patients with UCDs was 18.2%, and the most commonly seen UCD was asthma (3.4%) in our study, followed by neurologic disorders (3.3%) and obesity (2.6%). In Campbell et al.’s study, 24% of patients had at least one comorbidity, and the most common UCD was obesity, followed by neurologic diseases and asthma [22]. In Drouin et al.’s study, chronic encephalopathy with severe neurodisability and obesity were the leading UCDs, and asthma was second [23]. The reason for obesity not being the most common UCD in children with COVID-19 in our study may be explained by the relatively low incidence of obesity in the general population in Turkey compared with other countries [24].
UCDs may lead to severe clinical courses in COVID-19. In a Morbidity and Mortality Weekly Report of the CDC, among 121 patients who died of COVID-19, 91 (75%) were found to have an underlying medical condition [25]. No patients died on the study day in our study. On the other hand, hospitalized patients were found to have a substantially higher ratio of UCDs than outpatients. COVID-19 in children with comorbidities may be interpreted as requiring hospitalization more frequently than in children with COVID-19 without UCDs. Alternatively, the higher hospitalization rate among children with UCDs may be due to the tendency of physicians to admit patients with comorbidities. Choi et al. stated a similar approach in their study, which emphasized that hospitalization should be considered for children with comorbidities who were infected with SARS-CoV-2 due to the increased risk of severe infection in this population [26].
Levels of respiratory support differed significantly between patients with and without UCDs. Compared with those without UCDs, patients with UCDs received oxygen support and invasive mechanical ventilation more often and were observed in room air less often. In Tsankov et al.’s systematic review and meta-analysis, children with UCDs and children with obesity were found to have a higher risk of severe COVID-19 [27]. In another systematic review and meta-analysis published in February 2022, being a neonate, prematurity in young infants, obesity, diabetes, chronic lung disease, heart disease, neurologic disease, and immunocompromised status were found as significant risk factors for severe COVID-19 in children. It was also striking that children with neurologic disorders in general, and children with seizure disorders had higher rates of developing severe COVID-19 and requiring hospitalization [26]. Similarly, in our study, children with neurologic disorders had a significantly higher frequency of dyspnea, pneumoniae, respiratory support need, and PICU admission. Another systematic review and meta-analysis focused on the impact of asthma on COVID-19 severity. The authors revealed that the risk factor for hospitalization, critical care admission, and clinical severity did not differ in children with asthma with COVID-19 [28]. In our research, the rate of dyspnea, pneumoniae, respiratory support, and PICU need was not found to be statistically higher in children with asthmatic than in those without asthma.
The overall vaccination rate among the children in the entire study population was 12.5%. According to the decree of the Turkish Ministry of Health authorities, patients aged over 12 years have been given access to the COVID-19 vaccines that are available in Turkey. When the children aged over 12 years were examined, the ratio of patients vaccinated voluntarily was 38.7%. This ratio was relatively small, especially compared with the literature, considering it included all children with at least one dose of the COVID-19 vaccine. Esposito et al. reported that 80.4% of adolescents aged 11–17 years were vaccinated with at least two doses of a COVID-19 vaccine [29]. In a multi-country study from the Eastern Mediterranean Region, children’s rates of COVID‑19 vaccination were determined using parent reports and were detected at 38.7%. When patients with UCDs in our study were evaluated within the > 12 years vaccine-eligible subgroup, only patients with obesity had higher vaccination rates than patients without obesity. Although vaccination rates were estimated to be higher in patients with UCDs compared with children without UCDs, there was no propensity to be vaccinated in UCD groups, except in those with obesity.
In a prospective longitudinal cohort study conducted in the UK, symptom distribution was extensively analyzed in vaccinated versus unvaccinated children and adolescents aged 12–17 years. According to the study, when evaluating patients previously infected with SARS-CoV-2, most symptoms had lower prevalence in the vaccinated compared with the unvaccinated, in Delta and Omicron predominance [30]. Similarly, in our study, fever, diarrhea, and pneumoniae were seen at higher rates in the unvaccinated than in the vaccinated. However, surprisingly, the headache, sore throat, fatigue, and myalgia ratio was higher in the vaccinated than in the unvaccinated. This might be because COVID-19 primarily manifests as flu-like symptoms rather than a lower respiratory tract infection in COVID-19-vaccinated individuals.
Studies are showing less severe outcomes of COVID-19 after vaccination in adults [31, 32]. However, we could not encounter many pediatric studies investigating the relationship between disease severity and vaccination. In a literature review and meta-analysis by Zheng et al., COVID-19 vaccination was found to be protective against hospitalization in adults [33]. Although Zimmerman et al.’s review indicated that the prevention of SARS-CoV-2 infection was not as strong an argument for vaccinating all healthy children as it was for adults, inpatients were found to be less frequently vaccinated in our study [34]. In Delen and Örtekus retrospective cohort study, which examined the population aged over 18 years, mortality was found to be lower in patients who had received a second dose of the Sinovac vaccine compared with unvaccinated patients [31]. No patients died on February 2nd, 2020, in participant centers in our study. We did not determine the disease’s severity; however, pneumoniae was found to be statistically more frequent in the unvaccinated group. Among the unvaccinated, oxygen support and PICU needs were also greater than among the vaccinated, although it was not statistically significant. When including only patients aged 12 years, pneumoniae, oxygen support, and PICU need were higher among the unvaccinated than in the vaccinated. However, we could not show statistical significance.
As a point prevalence study, it includes several limitations. Considering the decreasing attitude regarding obtaining PCR results for COVID-19 from suspected cases in our country, the disease prevalence was estimated to be more than was determined. Therefore, the numbers and results might not reflect actual values. Also, because 1-day monitoring was the only source of patients’ information, as per the nature of point prevalence studies, the clinical course of the patients could not be followed. Moreover, we did not determine symptom distributions within age groups because this was not our study’s primary goal, which was mainly to assess hospitalization and UCD rates, as well as vaccination status. However, the clinical presentation of COVID-19 may vary among pediatric age groups. Lastly, variants of COVID-19 could not be analyzed in most centers. Even though in February 2022, Omicron was the leading variant for all cities in Turkey, as orally declared by the Turkish Health Minister, we could not determine variants in PCR analysis in most cities; clinical severity may differ among children infected with different COVID-19 variants. Despite its limitations, this study attempted to provide a snapshot of pediatric COVID-19 and the everyday activities of pediatric infectious diseases specialists. Therefore, we believe this study’s goal was reached because it provided an overlook of COVID-19 in children.
In conclusion, the overall hospitalization rate on February 2nd, 2022, in pediatric patients with COVID-19 was approximately 25%, and 1.8% of the patients were admitted to the PICU. The most common symptoms of the patients were fever and cough, and the most common UCD was asthma. Fever and pneumoniae rates were lower in vaccinated children. Nowadays, with the increased accessibility to COVID-19 vaccines, vaccination efforts against COVID-19 should be enhanced, particularly for children with UCDs.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- PCR:
-
Polymerase chain reaction
- PICU:
-
Pediatric intensive care unit
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- UCD:
-
Underlying chronic diseases
References
World Health Organization (WHO) (2022) Timeline: WHO’s COVID-19 response (WHO web site) 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline. Retrieved 10 Nov 2022
World Health Organization (WHO) (2022) Tracking SARS-CoV-2 variants (WHO web site) 2022. https://www.who.int/activities/tracking-SARS-CoV-2-variants. Retrieved 10 Nov 2022
Ludvigsson JF (2020) Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109:1088–1095. https://doi.org/10.1111/apa.15270
Du W, Yu J, Wang H, Zhang X, Zhang S, Li Q, Zhang Z (2020) Clinical characteristics of COVID-19 in children compared with adults in Shandong Province. China Infection 48(3):445–452. https://doi.org/10.1007/s15010-020-01427-2
Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M et al (2021) Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 22(1):43–55. https://doi.org/10.1016/S1473-3099(21)00460-6
Centers for Disease Control and Prevention (CDC) (2022) COVID-19 vaccination for children. https://www.cdc.gov/vaccines/covid-19/planning/children.html#covid19-vax-recommendations. Retrieved 10 Nov 2022
NTV (Turkish National News) (2022) 12 yaş üstü çocuklara 3. doz corona virüs aşısı başladı - Sağlık Haberleri. https://www.ntv.com.tr/saglik/12-yas-ustu-cocuklara-3-doz-corona-virus-asisi-basladi,V6Lz2bN99kmfqGhKp_WJJA. Retrieved 10 November 2022.
World Health Organization (WHO) (2022) Türkiye: WHO coronavirus disease (COVID-19) dashboard with vaccination data. https://covid19.who.int/region/euro/country/tr. Retrieved 10 Nov 2022
Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V et al (2020) COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 4(9):653–661. https://doi.org/10.1016/S2352-4642(20)30177-2
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S (2020) Epidemiology of COVID-19 among children in China. Pediatrics 145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
Li B, Zhang S, Zhang R, Chen X, Wang Y, Zhu C (2020) Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front Pediatr 8:591132. https://doi.org/10.3389/fped.2020.591132
Mantovani A, Rinaldi E, Zusi C, Beatrice G, Saccomani MD, Dalbeni A (2021) Coronavirus disease 2019 (COVID-19) in children and/or adolescents: a meta-analysis. Pediatr Res 89(4):733–737. https://doi.org/10.1038/s41390-020-1015-2
Assaker R, Colas AE, Julien-Marsollier F, Bruneau B, Marsac L, Greff B, Tri N, Fait C, Brasher C, Dahmani S (2020) Presenting symptoms of COVID-19 in children: a meta-analysis of published studies. Br J Anaesth 125(3):e330–e332. https://doi.org/10.1016/j.bja.2020.05.026
Christophers B, Gallo Marin B, Oliva R, Powell WT, Savage TJ, Michelow IC (2022) Trends in clinical presentation of children with COVID-19: a systematic review of individual participant data. Pediatr Res 91(3):494–501. https://doi.org/10.1038/s41390-020-01161-3
Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z (2021) Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child 106(5):440–448. https://doi.org/10.1136/archdischild-2020-321385
Wang Z, Zhao S, Tang Y, Wang Z, Shi Q, Dang X, Gan L, Peng S, Li W, Zhou Q et al (2022) Potentially effective drugs for the treatment of COVID-19 or MIS-C in children: a systematic review. Eur J Pediatr 181(5):2135–2146. https://doi.org/10.1007/s00431-022-04388-w
Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A, Abzug MJ, MacBrayne CE, Soma VL et al (2021) Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc 10(1):34–48. https://doi.org/10.1093/jpids/piaa115
Dulek DE, Fuhlbrigge RC, Tribble AC, Connelly JA, Loi MM, El Chebib H, Chandrakasan S, Otto WR, Diorio C, Keim G et al (2020) Multidisciplinary guidance regarding the use of immunomodulatory therapies for acute coronavirus disease 2019 in pediatric patients. J Pediatric Infect Dis Soc 9(6):716–737. https://doi.org/10.1093/jpids/piaa098
Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, Hu T, Li J, Zhou X, Ren B (2020) The microbial coinfection in COVID-19. Appl Microbiol Biotechnol 104(18):7777–7785. https://doi.org/10.1007/s00253-020-10814-6
Lansbury L, Lim B, Baskaran V, Lim WS (2020) Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 81(2):266–275. https://doi.org/10.1016/j.jinf.2020.05.046
Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP (2020) Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 26(10):1395–1399. https://doi.org/10.1016/j.cmi.2020.06.025
Campbell JI, Dubois MM, Savage TJ, Hood-Pishchany MI, Sharma TS, Petty CR, Lamb GS, Nakamura MM, Pediatric COVID-19 US Registry (2022) Comorbidities associated with hospitalization and progression among adolescents with symptomatic coronavirus disease 2019. J Pediatr 245:102–110.e2. https://doi.org/10.1016/j.jpeds.2022.02.048
Drouin O, Hepburn CM, Farrar DS, Baerg K, Chan K, Cyr C, Donner EJ, Embree JE, Farrell C, Forgie S et al (2021) Characteristics of children admitted to hospital with acute SARS-CoV-2 infection in Canada in 2020. CMAJ 193(38):E1483–E1493. https://doi.org/10.1503/cmaj.210053
Bereket A, Atay Z (2012) Current status of childhood obesity and its associated morbidities in Turkey. J Clin Res Pediatr Endocrinol 4(1):1–7. https://doi.org/10.4274/jcrpe.506
Bixler D, Miller AD, Mattison CP, Taylor B, Komatsu K, Peterson Pompa X, Moon S, Karmarkar E, Liu CY, Openshaw JJ et al (2020) SARS-CoV-2-associated deaths among persons aged <21 years - United States, February 12-July 31, 2020. MMWR Morb Mortal Wkly Rep 69(37):1324–1329. https://doi.org/10.15585/mmwr.mm6937e4
Choi JH, Choi SH, Yun KW (2022) Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. J Korean Med Sci 37(5):e35. https://doi.org/10.3346/jkms.2022.37.e35
Tsankov BK, Allaire JM, Irvine MA, Lopez AA, Sauvé LJ, Vallance BA, Jacobson K (2021) Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis 103:246–256. https://doi.org/10.1016/j.ijid.2020.11.163
Mongkonsritragoon W, Prueksapraoprong C, Kewcharoen J, Tokavanich N, Prasitlumkum N, Huang J, Poowuttikul P (2022) Prevalence and risk associated with asthma in children hospitalized with SARS-CoV-2: a meta-analysis and systematic review. J Allergy Clin Immunol Pract 10(5):1382-1384.e1. https://doi.org/10.1016/j.jaip.2021.12.044
Esposito S, Giordano R, Paini G, Puntoni M, Principi N, Caminiti C (2022) Can we get out of the COVID pandemic without adequate vaccination coverage in the pediatric population? Ital J Pediatr 48(1):150. https://doi.org/10.1186/s13052-022-01339-x
Molteni E, Canas LS, Kläser K, Deng J, Bhopal SS, Hughes RC, Chen L, Murray B, Kerfoot E, Antonelli M et al (2022) Post-vaccination infection rates and modification of COVID-19 symptoms in vaccinated UK school-aged children and adolescents: a prospective longitudinal cohort study. Lancet Reg Health Eur 19:100429. https://doi.org/10.1016/j.lanepe.2022.100429
Delen LA, Örtekus M (2022) Sinovac vaccination and the course of COVID-19 disease in hospitalized patients in Turkey. Ann Saudi Med 42(3):147–154. https://doi.org/10.5144/0256-4947.2022.147
Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, Fell DB, Austin PC, Schwartz KL, Sundaram ME et al (2022) Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol 7(3):379–385. https://doi.org/10.1038/s41564-021-01053-0
Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W (2022) Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis 114:252–260. https://doi.org/10.1016/j.ijid.2021.11.009
Zimmermann P, Pittet LF, Finn A, Pollard AJ, Curtis N (2022) Should children be vaccinated against COVID-19? Arch Dis Child 107(3):e1. https://doi.org/10.1136/archdischild-2021-323040
Acknowledgements
We would like to thank Mustafa Agah Tekindal for his valuable contribution to the statistical analyses of the study.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all the following: (1) the conception and design of the study, acquisition of data, or analysis and interpretation of data; (2) drafting of the article or critical review of it for important intellectual content; and (3) final approval of the submitted version.
Corresponding author
Ethics declarations
Ethics approval
The pediatric infectious disease clinic managed the study at Health Science University Izmir Tepecik Training and Research Hospital, where ethical approval was obtained with the 2021/09–15 decision number. Data from the centers were provided through e-mail, while ethical approval documents with wet signatures of each participant center were sent via mail to our hospital’s ethical committee unit. All investigational procedures conformed to the Declaration of Helsinki guiding principles.
Consent to participate
Not applicable.
Consent for publication
All the patient data are anonymized, and parents and legal guardians consented to publication of the results of the project.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yılmaz, D., Üstündağ, G., Büyükçam, A. et al. A snapshot of pediatric inpatients and outpatients with COVID-19: a point prevalence study from Turkey. Eur J Pediatr 182, 3231–3242 (2023). https://doi.org/10.1007/s00431-023-04982-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04982-6