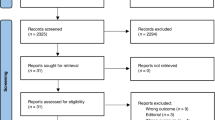
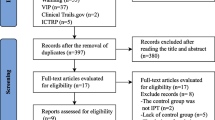
Abstract
Phototherapy is the main treatment of neonatal hyperbilirubinemia to prevent encephalopathy. It is generally believed to be safe; however, some studies have shown it might be associated with cancer development. In this systematic review and meta-analysis, we aimed to assess the effect of neonatal phototherapy on future cancer risk. A systematic search in 13 databases was conducted in December 2018 and updated in August 2022 to identify studies that report cancer development after exposure to phototherapy. Throughout the study period, regular manual searches were also conducted to include new studies. A meta-analysis using R programming language was done in which the odds ratios (ORs) with 95% confidence intervals (CIs) were estimated and pooled using the reported adjusted and unadjusted data. Fifteen studies were included. A statistically significant association was detected between neonatal phototherapy and any type of cancer (OR 1.24; 95% CI 1.1, 1.4), any hematopoietic cancer (OR 1.49; 95% CI 1.17, 1.91), any leukemia (OR 1.35; 95% CI 1.08, 1.67), and myeloid leukemia (OR 2.86; 95% CI 1.4, 5.84). The other investigated cancers (lymphoid leukemia, Hodgkin’s lymphoma, kidney cancer, nervous system cancer, and skin cancer) were not associated with phototherapy.
Conclusions: Phototherapy may carry a possible risk of future cancers. Future research is needed to quantify the magnitude of the cancer risk. These future studies should consider predictors of preterm birth or exclude premature babies from their analysis.
What is Known |
• There were various reports about the possible association between phototherapy in neonates and the increased risk of cancer in the future. |
What is New |
• A statistically significant association between phototherapy and various hematopoietic cancers (especially myeloid leukemia) was recorded. |
• The effect of the duration of phototherapy on the increased risk of hematopoietic cancers is yet unclear. |





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available. However, the data can be provided by the corresponding author (Nguyen Tien Huy, tienhuy@nagasaki-u.ac.jp) on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- GHL:
-
Global Health Library
- ICD:
-
International Classification of Diseases
- NHL:
-
Non-Hodgkin’s lymphoma
- NIH:
-
National Institutes of Health
- NYAM:
-
New York Academy of Medicine
- OR:
-
Odds ratio
- PRISMA:
-
Preferred Reporting Items for Systematic review and Meta-Analysis
- PT:
-
Phototherapy
- SD:
-
Standard deviation
- SIGLE:
-
System for Information on Grey Literature in Europe
- VHL:
-
Virtual Health Library
- WHO:
-
World Health Organization
References
Vreman HJ, Wong RJ, Stevenson DK (2004) Phototherapy: current methods and future directions. Semin Perinatol 28:326–333
Maisels MJ, McDonagh AF (2008) Phototherapy for neonatal jaundice. N Engl J Med 358:920–928
Maisels MJ (2001) Phototherapy--traditional and nontraditional. J Perinatol: official journal of the California Perinatal Association 21 Suppl 1:S93–97; discussion S104–107
Lightner DA, McDonagh AF (1984) Molecular mechanisms of phototherapy for neonatal jaundice. Acc Chem Res 17:417–424
Polin RA (1990) Management of neonatal hyperbilirubinemia: rational use of phototherapy. Biol Neonate 58(Suppl 1):32–43
Wang J, Guo G, Li A, Cai WQ, Wang X (2021) Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp Ther Med 21:231
Podvin D, Kuehn CM, Mueller BA, Williams M (2006) Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol 20:312–322
Cnattingius S, Zack M, Ekbom A, Gunnarskog J, Linet M, Adami HO (1995) Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 4:441–445
Steensel-Moll HA, Duijn CM, Valkenburg HA, Zanen GE (1992) Predominance of hospital deliveries among children with acute lymphocytic leukemia: speculations about neonatal exposure to fluorescent light. Cancer Causes Control 3:389–390
Cnattingius S, Zack MM, Ekbom A, Gunnarskog J, Kreuger A, Linet M, Adami HO (1995) Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Cancer Inst 87:908–914
Roman E, Ansell P, Bull D (1997) Leukaemia and non-Hodgkin’s lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer 76:406–415
Olsen JH, Hertz H, Kjaer SK, Bautz A, Mellemkjaer L, Boice JD Jr (1996) Childhood leukemia following phototherapy for neonatal hyperbilirubinemia (Denmark). Cancer causes & control : CCC 7:411–414
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372
Tawfik GM, Dila KAS, Mohamed MYF, Tam DNH, Kien ND, Ahmed AM, Huy NT (2019) A step by step guide for conducting a systematic review and meta-analysis with simulation data. Tropical medicine and health 47:46
Auger N, Laverdiere C, Ayoub A, Lo E, Luu TM (2019) Neonatal phototherapy and future risk of childhood cancer. Int J cancer
National Institutes of Health (2014) Quality assessment tool for observational cohort and cross-sectional studies. National Heart, Lung, and Blood Institute Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 5 Nov 2015
Leung A, Heal C, Perera M, Pretorius C (2015) A systematic review of patient-related risk factors for catheter-related thrombosis. J Thromb Thrombolysis 40:363–373
Valentine JC, Pigott TD, Rothstein HR (2010) How many studies do you need? A primer on statistical power for meta-analysis. Journal of Educational and Behavioral Statistics 35:215–247
Cummings P (2009) The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med 163:438–445
Greenland S, Thomas DC (1982) On the need for the rare disease assumption in case-control studies. Am J Epidemiol 116:547–553
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses Bmj 327:557–560
Dalton JE, Bolen SD, Mascha EJ (2016) Publication bias: the elephant in the review. Anesth Analg 123:812
Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB (2016) Neonatal phototherapy and infantile cancer. Pediatrics 137
Berg P, Lindelof B (1998) Is phototherapy in neonates a risk factor for malignant melanoma development? Pediatr Dermatol 15:250
Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW (2016) Retrospective cohort study of phototherapy and childhood cancer in Northern California. Pediatrics 137
Brewster DH, Tucker JS, Fleming M, Morris C, Stockton DL, Lloyd DJ, Bhattacharya S, Chalmers JW (2010) Risk of skin cancer after neonatal phototherapy: retrospective cohort study. Arch Dis Child 95:826–831
Ku M-S (2018) Neonatal phototherapy: a novel therapy to prevent allergic skin disease for at least 5 years. Neonatology 114:235–241
Sabzevari F, Sinaei R, Bahmanbijari B, Dehghan Krooki S, Dehghani A (2022) Is neonatal phototherapy associated with a greater risk of childhood cancers? BMC Pediatr 22:1–7
Kadivar M, Sangsari R, Saeedi M, Ghasemi Tehrani S (2020) Association between neonatal phototherapy and cancer during childhood. Iranian Journal of Neonatology IJN 11:104–108
Bugaiski-Shaked A, Shany E, Mesner O, Sergienko R, Wainstock T (2022) Association between neonatal phototherapy exposure and childhood neoplasm. J Pediatr
Digitale JC, Kim M-O, Kuzniewicz MW, Newman TB (2021) Update on phototherapy and childhood cancer in a Northern California cohort. Pediatrics 148
Hemati Z, Keikha M, Khoshhali M, Kelishadi R (2022) Phototherapy and risk of childhood cancer: a systematic review and meta-analysis. J Neonatal Nurs
Wintermeier K, von Poblotzki M, Genzel-Boroviczény O, Vogel S, Schotten K, Berking C, Giehl KA (2014) Neonatal blue light phototherapy increases café-au-lait macules in preschool children. Eur J Pediatr 173:1519–1525
Csoma Z, Toth-Molnar E, Balogh K, Polyanka H, Orvos H, Ocsai H, Kemeny L, Szell M, Olah J (2011) Neonatal blue light phototherapy and melanocytic nevi: a twin study. Pediatrics 128:e856-864
Mahé E, Beauchet A, Aegerter P, Saiag P (2009) Neonatal blue-light phototherapy does not increase nevus count in 9-year-old children. Pediatrics 123:e896–e900
Csoma Z, Hencz P, Orvos H, Kemeny L, Dobozy A, Dosa-Racz E, Erdei Z, Bartusek D, Olah J (2007) Neonatal blue-light phototherapy could increase the risk of dysplastic nevus development. Pediatrics 119:1269
Bauer J, Büttner P, Luther H, Wiecker TS, Möhrle M, Garbe C (2004) Blue light phototherapy of neonatal jaundice does not increase the risk for melanocytic nevus development. Arch Dermatol 140:493–494
Matichard E, Le Henanff A, Sanders A, Leguyadec J, Crickx B, Descamps V (2006) Effect of neonatal phototherapy on melanocytic nevus count in children. Arch Dermatol 142:1599–1604
Speck WT, Rosenkranz HS (1979) Phototherapy for neonatal hyperbilirubinemia—a potential environmental health hazard to newborn infants: a review. Environ Mutagen 1:321–336
Kahveci H, Dogan H, Karaman A, Caner I, Tastekin A, Ikbal M (2013) Phototherapy causes a transient DNA damage in jaundiced newborns. Drug Chem Toxicol 36:88–92
Spikes JD (1984) Photobiology of porphyrins. Prog Clin Biol Res 170:19–39
Buettner GR, Oberley LW (1979) Superoxide formation by protoporphyrin as seen by spin trapping. FEBS Lett 98:18–20
Evensen JF, Moan J (1982) Photodynamic action and chromosomal damage: a comparison of haematoporphyrin derivative (HpD) and light with X-irradiation. Br J Cancer 45:456–465
Karakukcu C, Ustdal M, Ozturk A, Baskol G, Saraymen R (2009) Assessment of DNA damage and plasma catalase activity in healthy term hyperbilirubinemic infants receiving phototherapy. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 680:12–16
El-Abdin MYZ, El-Salam MA, Ibrhim MY, Koraa SS, Mahmoud E (2012) Phototherapy and DNA changes in full term neonates with hyperbilirubinemia. Egypt J Med Hum Genet 13:29–35
Yahia S, Shabaan AE, Gouida M, El-Ghanam D, Eldegla H, El-Bakary A, Abdel-Hady H (2015) Influence of hyperbilirubinemia and phototherapy on markers of genotoxicity and apoptosis in full-term infants. Eur J Pediatr 174:459–464
Aycicek A, Kocyigit A, Erel O, Senturk H (2008) Phototherapy causes DNA damage in peripheral mononuclear leukocytes in term infants. J Pediatr 84:141–146
Harder T, Plagemann A, Harder A (2008) Birth weight and subsequent risk of childhood primary brain tumors: a meta-analysis. Am J Epidemiol 168:366–373
Caughey RW, Michels KB (2009) Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer 124:2658–2670
Heuch JM, Heuch I, Akslen LA, Kvåle G (1998) Risk of primary childhood brain tumors related to birth characteristics: a Norwegian prospective study. Int J Cancer 77:498–503
Bjørge T, Cnattingius S, Lie RT, Tretli S, Engeland A (2008) Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomark Prev 17:500–506
de Fine LS, Schmidt L, Rod N, Schmiegelow K, Lähteenmäki P, Kogner P, Träger C, Stokland T, Schüz J (2012) Hepatoblastoma in the Nordic countries. Int J Cancer 131:E555–E561
Bjørge T, Sørensen HT, Grotmol T, Engeland A, Stephansson O, Gissler M, Tretli S, Troisi R (2013) Fetal growth and childhood cancer: a population-based study. Pediatrics 132:e1265–e1275
Barahmani N, Dorak MT, Forman MR, Sprehe MR, Scheurer ME, Bondy ML, Okcu MF, Lupo PJ (2015) Evaluating the role of birth weight and gestational age on acute lymphoblastic leukemia risk among those of Hispanic ethnicity. Pediatr Hematol Oncol 32:382–389
Lupo PJ, Schraw JM, Desrosiers TA, Nembhard WN, Langlois PH, Canfield MA, Copeland G, Meyer RE, Brown AL, Chambers TM (2019) Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol 5:1150–1158
Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS (2004) Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res 56:682–689
Watchko JF (2021) Review of the contribution of genetic factors to hyperbilirubinemia and kernicterus risk in neonates: a targeted update. Pediatric Medicine 4
Lamola AA, Bhutani VK, Du L, Castillo Cuadrado M, Chen L, Shen Z, Wong RJ, Stevenson DK (2015) Neonatal bilirubin binding capacity discerns risk of neurological dysfunction. Pediatr Res 77:334–339
Hansen TWR, Wong RJ, Stevenson DK (2020) Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev 100:1291–1346
Kuzniewicz MW, Escobar GJ, Newman TB (2009) Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 124:1031–1039
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia (2004) Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114:297–316
Tyson JE, Pedroza C, Langer J, Green C, Morris B, Stevenson D, Van Meurs KP, Oh W, Phelps D, O’Shea M (2012) Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? J Perinatol 32:677–684
Hansen T (2012) Let there be light—but should there be less? J Perinatol 32:649–651
Newman TB, Kuzniewicz MW, Liljestrand P, Wi S, McCulloch C, Escobar GJ (2009) Numbers needed to treat with phototherapy according to American Academy of Pediatrics guidelines. Pediatrics 123:1352
Wu YW, Kuzniewicz MW, Wickremasinghe AC, Walsh EM, Wi S, McCulloch CE, Newman TB (2015) Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: a population-based study. JAMA Pediatr 169:239–246
Wickremasinghe AC, Risley RJ, Kuzniewicz MW, Wu YW, Walsh EM, Wi S, McCulloch CE, Newman TB (2015) Risk of sensorineural hearing loss and bilirubin exchange transfusion thresholds. Pediatrics 136:505–512
Acknowledgements
We are very grateful to Professor Thor Willy Ruud Hansen (t.w.r.hansen@medisin.uio.no) for his thorough revision and his contributions to improving the overall quality of the manuscript. We also thank Lina Hemmeda for her contributions to the previous version of the manuscript. Finally, we would like to express our gratitude to Dr. Julia Wang (wang.julia.m@gmail.com) for proofreading and editing the manuscript before its publication.
Author information
Authors and Affiliations
Contributions
NTH developed the idea. All authors screened the articles for eligibility and collected the data using the standardized extraction Excel sheet. MA, AMM, and NTH performed the data analysis. MA created the tables and figures. All authors contributed to writing the manuscript. All authors approved of the final version of the manuscript before submission for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdellatif, M., Tawfik, G.M., Makram, A.M. et al. Association between neonatal phototherapy and future cancer: an updated systematic review and meta-analysis. Eur J Pediatr 182, 329–341 (2023). https://doi.org/10.1007/s00431-022-04675-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04675-6