Abstract
Persistent pulmonary hypertension of the neonate (PPHN) refractory to inhaled nitric oxide still represents a frequent clinical challenge with negative outcomes in neonatal critical care. Several pulmonary vasodilators have become available thanks to improved understanding of pulmonary hypertension pathobiology. These drugs are commonly used in adults and there are numerous case series and small studies describing their potential usefulness in neonates, as well. New vasodilators act on different pathways, some of them can have additive effects and all have different pharmacology features. This information has never been summarized so far and no comprehensive pathobiology-driven algorithm is available to guide the treatment of refractory PPHN.
Conclusion: We offer a rational clinical algorithm to guide the treatment of refractory PPHN based on expert advice and the more recent pathobiology and pharmacology knowledge.
What is Known: • Refractory PPHN occurs in 30–40% of iNO-treated neonates and represents a significant clinical problem. Several pulmonary vasodilators have become available thanks to a better understanding of pulmonary hypertension pathobiology. What is New: • Available vasodilators have different pharmacology, mechanisms of action and may provide additive effect. We provide a rational clinical algorithm to guide the treatment of refractory PPHN based on expert advice and the more recent pathobiology and pharmacology knowledge. |
Similar content being viewed by others
Background
Persistent pulmonary hypertension of the neonate (PPHN) is a severe clinical syndrome representing the most common form of pediatric pulmonary hypertension and is usually associated with various types of respiratory failure [1]. Refractory PPHN is defined as an acutely life-threatening condition not responding to the administration of inhaled nitric oxide (iNO at the classical dose of 20 ppm) and conventional measures (including mechanical ventilation (with alveolar recruitment, if needed) and inotropic support) with resulting hypoxemia and represents a significant proportion (up to 30–40% [2]) of PPHN patients. Refractoriness to iNO depends on many factors such as genetic background, gestational age, underlying conditions, and co-interventions; depending on these factors only some patients can be considered as candidate for extra-corporeal life support [3].
As the knowledge about pathobiology of pulmonary hypertension has been increasing in the last years, several pulmonary vasodilators acting on different molecular pathways have been introduced for the treatment of pulmonary hypertension in adults. These drugs have been tested in numerous studies enrolling small newborn populations, thus strong evidence is unavailable due to difficulty in designing adequately powered, explanatory trials enrolling large, and homogeneous populations. However, these small studies have been accumulating over the years suggesting clinical benefits, supported by a strong pathobiological background. Refractory PPHN may represent an indication for extra-corporeal life support, but this may not be an option for all patients and may not be available in all centers. Therefore, a large weaponry of pulmonary vasodilators is available to be used on a case-by-case scenario, considering their pharmacological features and PPHN pathobiology, when a patient cannot be candidate to extra-corporeal life support or this is unavailable. To the best of our knowledge, no comprehensive pathobiology-driven algorithm is available so far to guide the treatment of refractory PPHN. Consistently, our aim is to propose a rational clinical algorithm to guide the treatment of refractory PPHN in neonatal intensive care units (NICU).
The available weaponry
Table 1 reports the currently marketed drugs that have been used to treat refractory PPHN [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. We might classify pulmonary vasodilators as primary or secondary: the former are represented by drugs primarily designed to treat pulmonary hypertension (e.g., iloprost, bosentan), while the latter consist of drugs originally used for other indications that may have a certain effect on pulmonary hypertension (e.g.: milrinone, alprostadil). Their pharmacology features are remarkably different, and the following general principles should be highlighted:
-
1.
iNO remains the first-line therapy based on large evidence-based data and European guidelines [3] and, therefore, it is approved for neonates ≥34 weeks’ gestation. However, many trials have demonstrated its safety also in more premature infants and, therefore, recent US consensus supports the use of iNO to treat acute hypoxia due to PPHN in preterm infants as well [54]. iNO generally has a good safety profile without side effects, is classically used at 20 ppm, as higher doses do not provide additional advantages, while lower doses may be equally effective in some cases.[3] iNO has no absolute contra-indication, beyond neonatal meta-hemoglobinemia.[55] For refractory PPHN, second-line drugs should be added on top of iNO, as significant additive effect may be obtained (see below).
-
2.
Local (nebulized) pulmonary vasodilators should be generally preferred as they directly reach the site of action reducing the risk of systemic side effects. Moreover, when PPHN is secondary to a parenchymal, severely restrictive disorder (such as, for instance, neonatal acute respiratory distress syndrome (NARDS) [56]), the drug will preferentially reach lung areas that have been recruited and therefore are well ventilated. Thus, the risk to increase intrapulmonary shunt, and to worsen the ventilation/perfusion mismatch, is lower with nebulized vasodilators. This risk is less cogent in lung development disorders or pulmonary hypoplasia due to genetic anomalies,[57] but, anyway a local pulmonary vasodilator can be preferred, as there is no advantage from systemic therapies which can more frequently provide generalized side effects. Modern vibrating mesh nebulizers provide satisfactory drug delivery both in conventional and high-frequency oscillatory ventilation, if placed proximal to the endotracheal tube on the inspiratory limb of the ventilator circuit [58]. The same setting can be efficaciously used during non-invasive ventilation, as well [59].
-
3.
In an extremely acute setting like refractory PPHN, orally administered drugs are less suitable, since they may need longer time to reach an effective systemic distribution and little to nothing is known about their intestinal absorption in neonates and particularly in preterm ones [39, 46]. Conversely, oral drugs can be useful in the weaning phase, when other vasodilators are being reduced, and a more long-lasting treatment may be needed. Some drugs are available in a pediatric syrup-like oral suspension: whenever possible, these should be preferred to those available only as tablets since these latter could not provide accurate dosing.
-
4.
Some drugs are known to have an additive effect due to different mechanisms of action on distinct pathways. This has been observed in various animal studies or clinical reports describing patients of any age [12,13,14, 20, 25, 28, 29, 31, 35, 37, 38, 60,61,62,63,64,65,66,67,68] and is of utmost importance for the choice of drugs to treat refractory PPHN (Fig.1). Obtaining a greater pulmonary vasodilation with no or minor systemic side effects should be the first therapeutic objective in patients with refractory PPHN in order to reduce the need of extra-corporeal life support, especially in patients generally considered poor candidates for this intervention (preterm neonates <34 weeks’ gestation or weighting <2500g). Once the critical phase is over, weaning should be provided by de-escalating the last introduced vasodilator and anyway avoiding any sudden interruption. Synergy is well-known for some drugs, while for others it has been inconsistently reported or may be expected only in some conditions. For instance, drugs acting on different pathways (i.e., iNO and milrinone or prostacyclins; bosentan and prostacyclins) usually ensure an additive effect, while those with the same second messenger (i.e., iNO and sildenafil or tadalafil; milrinone and adenosine or prostacyclins) may show synergy or not, depending on the pathobiology of that particular case. In other words, if acting on that pathway with a previous drug has been successful, one may argue that a second vasodilator might potentiate the effect; however, if the already used drug did not provide any benefit, it might be unlikely that adding another vasodilator on the same pathway would be efficacious. It is important to remember that PPHN is a syndrome that may be associated to several underlying conditions with a significantly different pathobiology. This is largely unknown, since clinical studies have enrolled non-homogeneous populations of neonates with PPHN secondary to different conditions, thus the clinical response is not always actually predictable. Alprostadil represents a particular case, because it has the peculiarity to be efficacious in keeping the ductus arteriosus open. Alprostadil may vasodilate pulmonary vessels binding EP4 receptors, but may have an opposite (vasoconstriction) effect through EP1 and EP3 receptors [69]. Therefore, it has a weaker and less consistent pulmonary vasodilatory action compared to the other drugs. However, it may be useful if a restrictive or recently closed ductus arteriosus is associated with right ventricular failure or pulmonary overflow (see below) [28, 70].
Additive effect of main pulmonary vasodilators used for refractory PPHN. Drugs may show synergy in terms of vasodilation and oxygenation, as they have different molecular mechanisms of action on distinct pathway. Green cases with “+” indicate addictive effect reported in pre-clinical studies and/or in clinical reports (in patients of any age; see text for more details). Yellow cases with “±” indicate that an addictive effect has been inconsistently reported or that it may be expected only in some conditions (see text for more details). White cases with “?” indicates lack of data about the possible synergy between those two drugs. “Prostacyclin and analogs” refer, respectively, to epoprostenol and beraprost, iloprost or treprostinil
The clinical algorythm
We propose a rational clinical algorithm based on the above-described considerations, according to the following priority: (1) the known synergy between some drugs and their pharmacology features, (2) the preference to nebulization over other administration routes, (3) the knowledge of biological mechanisms of PPHN and the clinical experience accumulated on the different drugs, and (4) the possible coexistence of particular hemodynamic conditions. For the algorithm application, careful monitoring is essential and should be provided with multiple vital parameters control (including perfusion index, arterial pressure, pre- and post-ductal saturation) and serial point-of-care echocardiography according to current international guidelines [71]. Some monitoring may need invasive techniques to be accurate. Additional monitoring techniques, such as electrical cardiometry or near-infrared spectroscopy might also be useful, if available.[72, 73]
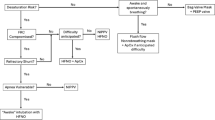
Figure 2 describes the proposed algorithm in detail. As first level, the basic management is obviously composed of iNO and hemodynamic support, that is, volume filling and norepinephrine, which are administered to reduce the pulmonary-systemic arterial pressure difference, reverse the shunt through the patent ductus arteriosus (if any) and support peripheral perfusion. Other inotropes (notably vasopressin, dobutamine or epinephrine) may also be needed and titrated, according to point-of-care echocardiography findings and monitoring of vital parameters. The treatment of underlying conditions such as NARDS or sepsis and optimal ventilatory support (including sedation and muscle paralysis, as needed) must be considered as basic management, as well. Hydrocortisone can also be considered in this phase for some patients such as preterm neonates or infants with septic shock. In fact, hydrocortisone may increase systemic pressure in preterm infants with relative cortico-adrenal insufficiency [74] and is included in the neonatal septic shock management [75]. Moreover, there are some data suggesting that it might provide benefits by increasing intracellular cyclic guanosine monophosphate and reducing inflammation associated with PPHN and several underlying conditions [76, 77].
Rational clinical algorithm to guide the treatment of refractory PPHN. The flow-chart is based on the principles described in the main text, considering the known synergy between the drugs, the preference to nebulization, the knowledge of biological mechanisms of PPHN, the clinical experience accumulated on the different drugs and the possible coexistence of particular hemodynamic conditions. Therapeutic interventions are categorized in 4 levels with different colors. Full lines indicate failure of the previous level treatment (i.e., persistence of life-threatening PPHN). Hatched lines indicate a therapeutic option when particular hemodynamic conditions coexist. Faded gray gradient and the big arrow depict an increasing risk of extra-corporeal life support for neonates who can be candidates to that. Extra-corporeal life support should not be delayed and instituted with the classical indications and oxygenation thresholds if the interventions proposed in the algorithm fail. The time to pass from one level to the next can be variable, however the effect of nebulized iloprost is always very quick (within minutes), while milrinone and alprostadil also provide relatively rapid (within few hours) vasodilation and oxygenation improvement, if any. Therefore, an iloprost nebulization trial can always be quickly done without delaying the further steps or extra-corporeal life support. Serial point-of-care echocardiography as internationally recommended [71] and/or other hemodynamic monitoring are essential at any step of the algorithm. *The basic management consists of the treatment of underlying conditions and best ventilatory support together with optimized hemodynamic management including iNO and volume filling and/or inotropes infusion as needed. Norepinephrine is usually administered to reduce the pulmonary arterial/systemic pressure difference and support peripheral perfusion. Other inotropes (notably vasopressin, epinephrine, dobutamine) may be added according to hemodynamic monitoring findings. Sedation, paralysis and hydrocortisone can also be considered within the basic management phase. Abbreviations: CDH: congenital diaphragmatic hernia; IV: intravenous
Iloprost, the carbacyclin analog of prostacyclin (i.e., prostaglandin-I2 (PGI2)), is preferred as second-line treatment after iNO failure, as it is a well-known vasodilator characterized by synergy with iNO, optimal safety profile and nebulization [12]. Iloprost potency is equal to that of iNO and has a fast action so the response (if any) can be quickly detected without delaying other treatments and extra-corporeal life support [78, 79]. Due to its short half-life, the time between iloprost administration may need to be shortened or a continuous nebulization may be possible, as it has been done for epoprostenol (which is the pharmaceutical form of PGI2) [23].
Milrinone, an ino-dilator able to increase myocardial contractility and reduce afterload, is considered after iloprost in case of impaired cardiac contractility, provided that related hypotension can be controlled with the basic management. As milrinone inhibits phosphodiesterase-3, it may also provide an additional synergic pulmonary vasodilation by reducing adenosine monophosphate breakdown and increasing its intracellular levels (Fig.1) [66]. Enoximone, another ino-dilator of the same family, has been used in only two cases, when milrinone was unavailable, and should not take its place due to the very limited experience [40, 53].
Alprostadil, the pharmaceutical form of prostaglandin-E2 (PGE2) is considered for some particular conditions characterized by the presence of restrictive or recently closed ductus arteriosus associated with: (1) right ventricular failure, or (2) pulmonary overflow with congested pulmonary veins. These are two particular and completely different scenarios, in which, however, alprostadil might be beneficial. The first (right ventricular failure) may occur in patients with very severe refractory PPHN (such as, patients with congenital diaphragmatic hernia). The second (pulmonary overflow with pulmonary veins congestion) is typical of patients with excessively early (that is, antenatal or early after birth) closure of the ductus arteriosus. In these two scenarios: (1) the right heart is not tolerating PPHN, or (2) lung edema occurs due to pulmonary overflow: in both cases opening the ductus may be helpful by downloading the right ventricle or reducing the pulmonary artery flow. Anyway, alprostadil has an inconsistent pulmonary vasodilator effect, and its use might also cause a marked increase of right-to-left shunt resulting in a worsening hypoxia, but it can help to tolerate PPHN while other drugs lower pulmonary pressures [69].
If PPHN remains life-threatening despite iNO and iloprost administration, bosentan (a potent dual endothelin receptor antagonist) is preferred as third-line treatment, despite it is only orally administered, since it shows additive effects with iNO and prostacyclins [37]. Moreover, the inhibition of endothelin pathway is strongly supported by specific biological data, since circulating levels of endothelin-1 are significantly higher in neonates with PPHN secondary to various conditions [80,81,82].
Adenosine is preferred as last line treatment, since it has an ideal safety profile and can have an additive effect with iNO, although neonatal experience on its use is smaller than that accumulated about iloprost and milrinone.
The majority of the described drugs are off-label in neonates. This is not surprising since more than 95% NICU-admitted neonates receive off-label drugs during their stay [83]. An extremely acute life-threatening condition such as refractory PPHN, justifies the use of these therapeutics, but demands a logical framework for their management and the proposed algorithm is useful to this purpose.
Sildenafil is not suggested in this context for several reasons. It is not free from side effects (Table 1) and the US Food and Drug Administration discouraged its use (especially at high dosages) in children beyond neonatal age [84]. More and above this, there are pharmacologic features making sildenafil less suitable for an extremely acute setting, such as refractory PPHN. In fact, the systemic availability of oral sildenafil in neonates is not well known and may not be reliably and quickly achieved to treat life-threatening PPHN [39]. Moreover, its renal clearance significantly increases during the first days of life and this influences the availability, as well [85]. On the other hand, the intravenous administration of sildenafil is not always available and is frequently associated with clinically significant systemic hypotension [86]. The vasodilatory potency of sildenafil is significantly lower than that of iloprost and it may not show synergy with iNO, as well as it can reduce the protective mechanism of hypoxic vasoconstriction in restrictive syndromes such as NARDS [5, 11, 22]. Conversely, sildenafil can be suitable in setting different from “acute” life-threatening refractory PPHN. For instance, short course sildenafil is useful when weaning iNO to avoid pulmonary pressure rebounds [87]. Similarly, sildenafil is valuable for chronic pulmonary hypertension due to bronchopulmonary dysplasia [88] or congenital heart disease [89] and has been associated to iloprost in non-invasively ventilated infants to spare the need of iNO and invasive ventilation [59, 90].
This algorithm is responding to an actual clinical unmet need but has also some limitations. The pathophysiology, biology, and pharmacology features of pulmonary vasodilators have not been resumed and integrated in a protocol to treat refractory PPHN so far, although these drugs are available worldwide. Randomized clinical trials are difficult to be conducted in this area, mainly because PPHN is a syndrome secondary to several underlying conditions receiving different co-interventions. Thus, while waiting for more evidence, a physiopathology and pathobiology-based approach is desirable. There is no test or tool to predict the response to any step of the algorithm and a full patient monitoring is needed. However, the effect of nebulized iloprost is always very quick (within minutes) and milrinone and alprostadil also provide rapid vasodilation and oxygenation improvement, if any. Therefore, an iloprost nebulization trial can always be quickly attempted and the algorithm can generally be applied over a short time-period (2–6 h). Therefore, the algorithm is not designed to delay extra-corporeal life support: this, or at least a prompt referral system, should always be available. For those neonates who may be candidate to extra-corporeal life support, a careful evaluation should be done by expert neonatal intensivists through each different steps of the algorithm. The algorithm needs significant expertise in neonatal critical care, and particularly in hemodynamic monitoring, thus it may not be easily applied in settings lacking expertise or adequate monitoring tools: in these cases, a prompt referral to a more expert center must be ensured.
In conclusion, we offer a rational, integrated, pathobiology, and physiology-based protocol for the treatment of refractory PPHN. The algorithm is an example of personalized approach to a complex life-threatening problem that has a common clinical appearance (i.e., refractory hypoxia) but different possible causes. A personalized medicine strategy is likely to be more effective that a simplified “one drug fits all” approach. In our experience, the protocol has proven useful to guide the successful management to these critical cases and for teaching purposes. The protocol is based on availability of several drugs and should be adapted for those setting where not all these drugs are available. Some of them may be quite easily exchanged with others (for instance, epoprostenol or trepostinil can be used at the place of iloprost). Nonetheless, the protocol may be difficult to apply in low-resources settings where drugs and full monitoring are unavailable and specific protocols should be built for these particular settings.
Data Availability
N/A
Code availability
N/A
Abbreviations
- iNO:
-
inhaled nitric oxide
- NARDS:
-
neonatal acute respiratory distress syndrome
- NICU:
-
neonatal intensive care units
- PGE2 :
-
prostaglandin-E2
- PGI2 :
-
prostaglandin-I2 (i.e., prostacyclin)
- PPHN:
-
persistent pulmonary hypertension of the neonate
References
Rosenzweig EB, Abman SH, Adatia I et al (2019) Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 53:1801916. https://doi.org/10.1183/13993003.01916-2018
Dillard J, Perez M, Chen B (2020) Therapies that enhance pulmonary vascular NO-signaling in the neonate. Nitric Oxide 95:45–54. https://doi.org/10.1016/j.niox.2019.12.003
Macrae DJ, Field D, Mercier J-C et al (2004) Inhaled nitric oxide therapy in neonates and children: reaching a European consensus. Intensive Care Med 30:372–380. https://doi.org/10.1007/s00134-003-2122-3
Aboudi D, Swaminathan N, Brumberg H et al (2018) Sildenafil and Retinopathy of Prematurity in Preterm Infants with Bronchopulmonary Dysplasia. J Pediatr 199:16–21. https://doi.org/10.1016/j.jpeds.2018.04.005
Al Omar S, Salama H, Al Hail M et al (2016) Effect of early adjunctive use of oral sildenafil and inhaled nitric oxide on the outcome of pulmonary hypertension in newborn infants A feasibility study. J Neonatal Perinatal Med 9:251–259. https://doi.org/10.3233/NPM-16161
Avila-Alvarez A, Bravo-Laguna MC, Bronte LD, Cerro MJD (2013) Inhaled iloprost as a rescue therapy for transposition of the great arteries with persistent pulmonary hypertension of the newborn. Pediatr Cardiol 34:2027–2029. https://doi.org/10.1007/s00246-012-0575-2
Bassler D, Choong K, McNamara P, Kirpalani H (2006) Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Neonatology 89:1–5. https://doi.org/10.1159/000088192
Berger-Caron F, Piedboeuf B, Morissette G et al (2019) Inhaled Epoprostenol for Pulmonary Hypertension Treatment in Neonates: A 12-Year Experience. Am J Perinatol 36:1142–1149. https://doi.org/10.1055/s-0038-1676483
Darland LK, Dinh KL, Kim S et al (2017) Evaluating the safety of intermittent intravenous sildenafil in infants with pulmonary hypertension: safety of intermittent intravenous sildenafil in infants. Pediatr Pulmonol 52:232–237. https://doi.org/10.1002/ppul.23503
De Jaegere APMC, van den Anker JN (1998) Endotracheal instillation of prostacyclin in preterm infants with persistent pulmonary hypertension. Eur Respir J 12:932–934. https://doi.org/10.1183/09031936.98.12040932
De Luca D, Zecca E, Vento G et al (2006) Transient effect of epoprostenol and sildenafil combined with iNO for pulmonary hypertension in congenital diaphragmatic hernia. Paediatr Anesth 16:597–598. https://doi.org/10.1111/j.1460-9592.2006.01879.x
De Luca D, Zecca E, Piastra M, Romagnoli C (2007) Iloprost as ?rescue? therapy for pulmonary hypertension of the neonate. Paediatr Anesth 17:394–395. https://doi.org/10.1111/j.1460-9592.2006.02104.x
El-Ghandour M, Hammad B, Ghanem M, Antonios MAM (2020) Efficacy of Milrinone Plus Sildenafil in the Treatment of Neonates with Persistent Pulmonary Hypertension in Resource-Limited Settings: Results of a Randomized, Double-Blind Trial. Pediatr Drugs 22:685–693. https://doi.org/10.1007/s40272-020-00412-4
Fatima N, Arshad S, Quddusi AI et al (2018) Comparison Of The Efficacy Of Sildenafil Alone Versus Sildenafil Plus Bosentan In Newborns With Persistent Pulmonary Hypertension. J Ayub Med Coll Abbottabad 30:333–336
Gaffuri M, Cristofaletti A, Mansoldo C, Biban P (2014) Acute onset of bilateral visual loss during sildenafil therapy in a young infant with congenital heart disease. BMJ Case Rep 2014:bcr2014204262. https://doi.org/10.1136/bcr-2014-204262
Giaccone A, Zuppa A, Sood B et al (2017) Milrinone Pharmacokinetics and Pharmacodynamics in Neonates with Persistent Pulmonary Hypertension of the Newborn. Am J Perinatol 34:749–758. https://doi.org/10.1055/s-0036-1597996
Goissen C, Ghyselen L, Tourneux P et al (2008) Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan. Eur J Pediatr 167:437–440. https://doi.org/10.1007/s00431-007-0531-y
Gupta N, Kamlin CO, Cheung M et al (2013) Prostaglandin E1 use during neonatal transfer: potential beneficial role in persistent pulmonary hypertension of the newborn. Arch Dis Child Fetal Neonatal Ed 98:F186–F188. https://doi.org/10.1136/archdischild-2012-303294
Iannotta R, Tana M, Priolo F et al (2020) Rectal bleeding in extremely preterm infants associated with oral sildenafil therapy: A case series. J Paediatr Child Health 56:163–164. https://doi.org/10.1111/jpc.14571
Iwamoto Y, Tamai A, Kawasaki H et al (2011) Late clinical manifestations of mitral valve disease and severe pulmonary hypertension in a patient diagnosed with premature closure of foramen ovale during fetal life. World J Pediatr 7:182–184. https://doi.org/10.1007/s12519-011-0276-6
Janjindamai W, Thatrimontrichai A, Maneenil G et al (2013) Effectiveness and safety of intravenous iloprost for severe persistent pulmonary hypertension of the newborn. Indian Pediatr 50:934–938. https://doi.org/10.1007/s13312-013-0263-1
Kahveci H, Yilmaz O, Avsar UZ et al (2014) Oral sildenafil and inhaled iloprost in the treatment of pulmonary hypertension of the newborn: Sildenafil and Iloprost in the Treatment of PH. Pediatr Pulmonol 49:1205–1213. https://doi.org/10.1002/ppul.22985
Kelly LK, Porta NFM, Goodman DM et al (2002) Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr 141:830–832. https://doi.org/10.1067/mpd.2002.129849
Kipfmueller F, Schroeder L, Berg C et al (2018) Continuous intravenous sildenafil as an early treatment in neonates with congenital diaphragmatic hernia. Pediatr Pulmonol 53:452–460. https://doi.org/10.1002/ppul.23935
Kodama Y, Tao K, Ishida F et al (2012) Long survival of congenital alveolar capillary dysplasia patient with NO inhalation and epoprostenol: effect of sildenafil, beraprost and bosentan: alveolar capillary dysplasia. Pediatr Int 54:923–926. https://doi.org/10.1111/j.1442-200X.2012.03712.x
Konduri GG, Garcia DC, Kazzi NJ, Shankaran S (1996) Adenosine infusion improves oxygenation in term infants with respiratory failure. Pediatrics 97:295–300
Lawrence KM, Berger K, Herkert L et al (2019) Use of prostaglandin E1 to treat pulmonary hypertension in congenital diaphragmatic hernia. J Pediatr Surg 54:55–59. https://doi.org/10.1016/j.jpedsurg.2018.10.039
Le Duc K, Mur S, Sharma D et al (2020) Prostaglandin E1 in infants with congenital diaphragmatic hernia (CDH) and life-threatening pulmonary hypertension. J Pediatr Surg 55:1872–1878. https://doi.org/10.1016/j.jpedsurg.2020.01.008
Maneenil G, Thatrimontrichai A, Janjindamai W, Dissaneevate S (2018) Effect of bosentan therapy in persistent pulmonary hypertension of the newborn. Pediatr Neonatol 59:58–64. https://doi.org/10.1016/j.pedneo.2017.02.003
McNamara PJ, Shivananda SP, Sahni M et al (2013) Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr Crit Care Med 14:74–84. https://doi.org/10.1097/PCC.0b013e31824ea2cd
Motti A, Tissot C, Rimensberger PC et al (2006) Intravenous adenosine for refractory pulmonary hypertension in a low-weight premature newborn: a potential new drug for rescue therapy. Pediatr Crit Care Med 7:380–382. https://doi.org/10.1097/01.PCC.0000225000.78627.EB
Nakwan N, Nakwan N, Wannaro J (2011) Persistent pulmonary hypertension of the newborn successfully treated with beraprost sodium: a retrospective chart review. Neonatology 99:32–37. https://doi.org/10.1159/000298137
Ng C, Franklin O, Vaidya M et al (2004) Adenosine infusion for the management of persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med 5:10–13. https://doi.org/10.1097/01.CCM.0000105309.27519.27
Patole S, Lee J, Whitehall J (1998) Adenosine infusion in the management of a micropremi neonate with pulmonary hypertension. Indian Pediatr 35:1221–1224
Park BY, Chung S-H (2017) Treprostinil for persistent pulmonary hypertension of the newborn, with early onset sepsis in preterm infant: 2 Case reports. Medicine (Baltimore) 96:e7303. https://doi.org/10.1097/MD.0000000000007303
Pawar R, Kasar P, Garekar S, Kulkarni S (2009) Use of Bosentan in neonatal post cardiac surgery pulmonary hypertension. Ann Pediatr Card 2:173. https://doi.org/10.4103/0974-2069.58325
Radicioni M, Bruni A, Camerini P (2011) Combination therapy for life-threatening pulmonary hypertension in a premature infant: first report on bosentan use. Eur J Pediatr 170:1075–1078. https://doi.org/10.1007/s00431-011-1422-9
Rugolotto S, Errico G, Beghini R et al (2006) Weaning of epoprostenol in a small infant receiving concomitant bosentan for severe pulmonary arterial hypertension secondary to bronchopulmonary dysplasia. Minerva Pediatr 58:491–494
Samiee-Zafarghandy S, Smith PB, van den Anker JN (2014) Safety of Sildenafil in Infants. Pediatr Crit Care Med 15:362–368. https://doi.org/10.1097/PCC.0000000000000077
Schranz D, Huth R, Michel-Behnke I, Wippermann C-F (1995) Norepinephrine, enoximone, and nitric oxide for treatment of myocardial stunning and pulmonary hypertension in a newborn with diaphragmatic hernia. J Pediatr Surg 30:801–804. https://doi.org/10.1016/0022-3468(95)90751-3
Shiyanagi S, Okazaki T, Shoji H et al (2008) Management of pulmonary hypertension in congenital diaphragmatic hernia: nitric oxide with prostaglandin-E1 versus nitric oxide alone. Pediatr Surg Int 24:1101–1104. https://doi.org/10.1007/s00383-008-2225-6
Soditt V, Aring C, Groneck P (1997) Improvement of oxygenation induced by aerosolized prostacyclin in a preterm infant with persistent pulmonary hypertension of the newborn. Intensive Care Med 23:1275–1278. https://doi.org/10.1007/s001340050498
Sood BG, Delaney-Black V, Aranda JV, Shankaran S (2004) Aerosolized PGE1: A Selective Pulmonary Vasodilator in Neonatal Hypoxemic Respiratory Failure Results of a Phase I/II Open Label Clinical Trial. Pediatr Res 56:579–585. https://doi.org/10.1203/01.PDR.0000139927.86617.B6
Steiner M, Salzer U, Baumgartner S et al (2014) Intravenous Sildenafil i. v. as Rescue Treatment for Refractory Pulmonary Hypertension in Extremely Preterm Infants. Klin Padiatr 226:211–215. https://doi.org/10.1055/s-0034-1375697
Steinhorn RH, Kinsella JP, Pierce C et al (2009) Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr 155:841–847.e1. https://doi.org/10.1016/j.jpeds.2009.06.012
Steinhorn RH, Fineman J, Kusic-Pajic A et al (2016) Bosentan as Adjunctive Therapy for Persistent Pulmonary Hypertension of the Newborn: Results of the Randomized Multicenter Placebo-Controlled Exploratory Trial. J Pediatr 177:90–96.e3. https://doi.org/10.1016/j.jpeds.2016.06.078
Stocker C, Penny DJ, Brizard CP et al (2003) Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Med 29:1996–2003. https://doi.org/10.1007/s00134-003-2016-4
Stultz JS, Puthoff T, Backes C, Nahata MC (2013) Intermittent intravenous sildenafil for pulmonary hypertension management in neonates and infants. Am J Health Syst Pharm 70:407–413. https://doi.org/10.2146/ajhp120364
Suzuki H, Sato S, Tanabe S, Hayasaka K (2002) Beraprost sodium for pulmonary hypertension with congenital heart disease. Pediatr Int 44:528–529. https://doi.org/10.1046/j.1442-200X.2002.01597.x
Tohyama M, Baba A, Tsuno T et al (1993) Aneurysmal dilatation of ductus arteriosus during lipo-prostaglandin E1 therapy for diaphragmatic hernia. Eur J Pediatr 152:877–879. https://doi.org/10.1007/BF01957520
Turbenson MN, Radosevich JJ, Manuel V, Feldman J (2020) Transitioning From Intravenous to Subcutaneous Prostacyclin Therapy in Neonates With Severe Pulmonary Hypertension. J Pediatr Pharmacol Ther 25:647–653. https://doi.org/10.5863/1551-6776-25.7.647
Iijima S, Ueno D, Baba T, Ohishi A (2018) Hypertrophic pyloric stenosis following persistent pulmonary hypertension of the newborn: a case report and literature review. BMC Pediatr 18:290. https://doi.org/10.1186/s12887-018-1270-0
van der Lee R, Peels B, Koopman-Esseboom C (2017) PDE3 inhibition with enoximone as first-line therapy for severe persistent pulmonary hypertension of the newborn during neonatal transport: a case report. Clin Case Rep 5:18–21. https://doi.org/10.1002/ccr3.748
Kinsella JP, Steinhorn RH, Krishnan US et al (2016) Recommendations for the Use of Inhaled Nitric Oxide Therapy in Premature Newborns with Severe Pulmonary Hypertension. J Pediatr 170:312–314. https://doi.org/10.1016/j.jpeds.2015.11.050
Centorrino R, Shankar-Aguilera S, Foligno S, De Luca D (2019) Life-threatening extreme methemoglobinemia during standard dose nitric oxide therapy. Neonatology 116:295–298. https://doi.org/10.1159/000501462
De Luca D, van Kaam AH, Tingay DG et al (2017) The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med 5:657–666. https://doi.org/10.1016/S2213-2600(17)30214-X
Galambos C, Mullen MP, Shieh JT et al (2019) Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur Respir J 54:1801965. https://doi.org/10.1183/13993003.01965-2018
DiBlasi RM, Crotwell DN, Shen S et al (2016) Iloprost Drug Delivery during Infant Conventional and High-Frequency Oscillatory Ventilation. Pulm Circ 6:63–69. https://doi.org/10.1086/685080
Piastra M, De Luca D, De Carolis MP et al (2012) Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr Pulmonol 47:757–762. https://doi.org/10.1002/ppul.21619
Nehra A, Blute ML, Barrett DM, Moreland RB (2002) Rationale for combination therapy of intraurethral prostaglandin E1 and sildenafil in the salvage of erectile dysfunction patients desiring noninvasive therapy. Int J Impot Res 14:S38–S42. https://doi.org/10.1038/sj.ijir.3900795
Liang F, Yang S, Yao L et al (2012) Ambrisentan and tadalafil synergistically relax endothelin-induced contraction of rat pulmonary arteries. Hypertension 59:705–711. https://doi.org/10.1161/HYPERTENSIONAHA.111.182261
Ghofrani HA, Wiedemann R, Rose F et al (2002) Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary Hypertension. Ann Intern Med 136:515. https://doi.org/10.7326/0003-4819-136-7-200204020-00008
Shekerdemian LS, Ravn HB, Penny DJ (2004) Interaction between inhaled nitric oxide and intravenous sildenafil in a porcine model of meconium aspiration syndrome. Pediatr Res 55:413–418. https://doi.org/10.1203/01.PDR.0000112033.81970.C2
Knebel SM, Elrick MM, Bowles EA et al (2013) Synergistic effects of prostacyclin analogs and phosphodiesterase inhibitors on cyclic adenosine 3′,5′ monophosphate accumulation and adenosine 3′5′ triphosphate release from human erythrocytes. Exp Biol Med (Maywood) 238:1069–1074. https://doi.org/10.1177/1535370213498981
Austin MJ, McDougall NI, Wendon JA et al (2008) Safety and efficacy of combined use of sildenafil, bosentan, and iloprost before and after liver transplantation in severe portopulmonary hypertension: combination therapy in PPHTN. Liver Transpl 14:287–291. https://doi.org/10.1002/lt.21310
Deb B, Bradford K, Pearl RG (2000) Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med 28:795–799. https://doi.org/10.1097/00003246-200003000-00031
McLaughlin V, Channick RN, Ghofrani H-A et al (2015) Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 46:405–413. https://doi.org/10.1183/13993003.02044-2014
Lakshminrusimha S, Porta NFM, Farrow KN et al (2009) Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med 10:106–112. https://doi.org/10.1097/PCC.0b013e3181936aee
Gomez I, Foudi N, Longrois D, Norel X (2013) The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot Essent Fat Acids 89:55–63. https://doi.org/10.1016/j.plefa.2013.04.004
Ly LG, Hawes J, Whyte HE et al (2007) The hemodynamically significant ductus arteriosus in Critically Ill Full-Term Neonates. Neonatology 91:260–265. https://doi.org/10.1159/000098173
Singh Y, Tissot C, Fraga MV et al (2020) International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 24(1):65. https://doi.org/10.1186/s13054-020-2787-9
Boet A, Jourdain G, Demontoux S, De Luca D (2016) Stroke volume and cardiac output evaluation by electrical cardiometry: accuracy and reference nomograms in hemodynamically stable preterm neonates. J Perinatol 36:748–752. https://doi.org/10.1038/jp.2016.65
Gagnon M-H, Wintermark P (2016) Effect of persistent pulmonary hypertension on brain oxygenation in asphyxiated term newborns treated with hypothermia. J Matern Fetal Neonatal Med 29:2049–2055. https://doi.org/10.3109/14767058.2015.1077221
Fernandez EF, Watterberg KL (2009) Relative adrenal insufficiency in the preterm and term infant. J Perinatol 29(Suppl 2):S44–S49. https://doi.org/10.1038/jp.2009.24
Wynn JL, Wong HR (2010) Pathophysiology and treatment of septic shock in neonates. Clin Perinatol 37:439–479. https://doi.org/10.1016/j.clp.2010.04.002
Perez M, Lakshminrusimha S, Wedgwood S et al (2012) Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. Am J Phys Lung Cell Mol Phys 302:L595–L603. https://doi.org/10.1152/ajplung.00145.2011
Alsaleem M, Malik A, Lakshminrusimha S, Kumar VH (2019) Hydrocortisone improves oxygenation index and systolic blood pressure in term infants with persistent pulmonary hypertension. Clin Med Insights Pediatr 13:117955651988891. https://doi.org/10.1177/1179556519888918
Mulligan C, Beghetti M (2012) Inhaled iloprost for the control of acute pulmonary hypertension in children: a systematic review. Pediatr Crit Care Med 13:472–480. https://doi.org/10.1097/PCC.0b013e31822f192b
Rimensberger PC, Spahr-Schopfer I, Berner M et al (2001) Inhaled nitric oxide versus aerosolized iloprost in secondary pulmonary hypertension in children with congenital heart disease: vasodilator capacity and cellular mechanisms. Circulation 103:544–548. https://doi.org/10.1161/01.CIR.103.4.544
Keller RL, Tacy TA, Hendricks-Munoz K et al (2010) Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med 182:555–561. https://doi.org/10.1164/rccm.200907-1126OC
Kumar P, Kazzi N, Shankaran S (1996) Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. Am J Perinatol 13:335–341. https://doi.org/10.1055/s-2007-994352
Rosenberg AA, Kennaugh J, Koppenhafer SL et al (1993) Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr 123:109–114. https://doi.org/10.1016/S0022-3476(05)81552-5
de Costa HTML, Costa TX, Martins RR, Oliveira AG (2018) Use of off-label and unlicensed medicines in neonatal intensive care. PLoS One 13:e0204427. https://doi.org/10.1371/journal.pone.0204427
http://www.fda.gov/Drugs/DrugSafety/ucm317123.htm [accessed on february 6, 2021]
Mukherjee A, Dombi T, Wittke B, Lalonde R (2009) Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther 85:56–63. https://doi.org/10.1038/clpt.2008.177
Lakshminrusimha S, Mathew B, Leach CL (2016) Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin Perinatol 40:160–173. https://doi.org/10.1053/j.semperi.2015.12.004
Namachivayam P, Theilen U, Butt WW et al (2006) Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med 174:1042–1047. https://doi.org/10.1164/rccm.200605-694OC
Mourani PM, Sontag MK, Ivy DD, Abman SH (2009) Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 154:379–384.e2. https://doi.org/10.1016/j.jpeds.2008.09.021
Cohen JL, Nees SN, Valencia GA, Rosenzweig EB, Krishnan US (2019) Sildenafil use in children with pulmonary hypertension. J Pediatr 205:29–34.e1. https://doi.org/10.1016/j.jpeds.2018.09.067
Gürakan B, Kayıran P, Öztürk N et al (2011) Therapeutic combination of sildenafil and iloprost in a preterm neonate with pulmonary hypertension. Pediatr Pulmonol 46:617–620. https://doi.org/10.1002/ppul.21415
Mizuno M, Aso K, Tsuzuki Y et al (2020) A successful treatment of tadalafil in incontinentia pigmenti with pulmonary hypertension. Eur J Med Genet 63:103764. https://doi.org/10.1016/j.ejmg.2019.103764
Funding
None
Author information
Authors and Affiliations
Contributions
Dr. Fortas wrote the first manuscript draft and performed literature search. Dr. Di Nardo substantially contributed to the literature search and in data interpretation and analysis. Dr. Yousef substantially contributed to the literature search and in data interpretation and analysis. Prof. Humbert substantially contributed to the literature search and in data interpretation and analysis. Prof. De Luca substantially contributed to the literature search and to data interpretation and analysis, synthetized the data and prepared the whole iconography, conceived and supervised the whole project. All authors reviewed the manuscript for important intellectual content, agreed to be accountable for all aspects of the work and approved the final manuscript version to be submitted.
Corresponding author
Ethics declarations
Ethical approval
N/A
Consent to participate
N/A
Consent for publication
N/A
Conflict of interest/Competing interests
Prof. Humbert reports personal fees from Acceleron, grants and personal fees from Actelion, grants and personal fees from Bayer, personal fees from GSK, personal fees from Merck, personal fees from Novartis, personal fees from Astrazeneca, personal fees from Sanofi, outside the submitted work The other authors have no conflict of interest or competing interest to declare.
Additional information
Communicated by Piet Leroy
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fortas, F., Di Nardo, M., Yousef, N. et al. Life-threatening PPHN refractory to nitric oxide: proposal for a rational therapeutic algorithm. Eur J Pediatr 180, 2379–2387 (2021). https://doi.org/10.1007/s00431-021-04138-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04138-4