Abstract
The use of supra-physiological, exogenous corticosteroids in pregnancy may lead to neonatal adrenal suppression. We report on a single-center, case series study carried out between 2006 and 2014, which included all newborns (n = 16) of mothers using prednisolone ≥10 mg/day during pregnancy. Newborns were routinely assessed according to hospital protocol, with follow-up until 6 weeks after birth. We investigated the clinical symptoms and biochemical findings of adrenal suppression occurring in the newborns. Mean dose of maternal prednisolone was 29.7 ± 16.1 mg/day with a mean duration of 18.4 ± 15.4 weeks. Five newborns showed hypoglycemia with normal serum cortisol concentrations and urinary steroid profiles. Two newborns had abnormal urinary steroid profiles, probably the result of prematurity, but with adequate adrenal stress response during clinical sepsis.
Conclusion: In this retrospective case series, we found no evidence of prolonged effects of maternal prednisolone use during pregnancy on the neonatal hypothalamic-pituitary-adrenal axis.
What is known: • The use of prednisolone during pregnancy may cause increased steroid levels in the fetus by partially passing through the placenta. • So far, there was very limited data available on the occurrence of adrenal suppression in the newborn of mothers using prednisolone during pregnancy. | |
What is new: • The use of high-dosage prednisolone during pregnancy for ≥1 week (mean duration of 18.4 ± 15.4 weeks), prior to delivery, appears to have little influence on the neonatal hypothalamic-pituitary-adrenal axis. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction in women with serious medical problems, such as chronic inflammatory bowel disease or asthma, has become increasingly common. The associated use of medication, including systemic corticosteroids, in pregnant women may incur an increased risk for the fetus or newborn baby.
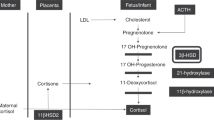
Prednisolone is the drug of choice as the estimated fetal level is only 10% of the maternal level [6]. This is the result of the conversion of prednisolone to less active metabolites by the enzyme 11β-hydroxysteroid dehydrogenase-2 (11-beta-HSD2) in the placenta [6]. There is less wide experience with the use of hydrocortisone during pregnancy. Dexamethasone is only partially metabolized by the enzyme 11-beta-HSD2, and up to 50% reaches the fetus [6]. When taken in high doses and over a longer period of time, prednisolone can saturate the placental enzymes, after which, larger amounts of corticosteroids will cross the placental barrier [1].
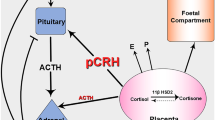
Chronic use of corticosteroids may result in suppressive effects on the neonatal hypothalamic-pituitary-adrenal (HPA) axis [8]. Then, in times of physical stress, such as an infection, the adrenal gland may not be able to produce sufficient hormone (cortisol) to help the newborn to respond adequately. The lack of this response (Addisonian crisis) is a life-threatening situation for which supplementation of corticosteroids is needed.
So far, there was no clinical data on the risk of HPA axis suppression in newborns whose mothers had been using systemic corticosteroids for a longer period during pregnancy. Neither were there any studies on the influence of dosage and duration of maternal prednisolone use during pregnancy. Local guidelines for monitoring HPA axis suppression in newborns were therefore developed in the UMCG, covering admission to the pediatric ward or neonatal intensive care unit (NICU), assessment of blood pressure, blood glucose levels, serum cortisol and plasma adrenocorticotropic hormone (ACTH) levels, urinary steroid profile (USP), and, if necessary, dynamic testing of the HPA axis. These measures lead to a distressing separation of mother and child and pose a substantial financial burden on the healthcare budget. Based on the current pathophysiological knowledge, we would expect a substantial number of cases to show HPA axis suppression. However, our clinical experience is that its prevalence, and that of refractory hypoglycemia and hypotension, is very low.
Our aim was to determine the clinical symptoms and biochemical signs of HPA axis suppression occurring in newborns whose mothers had used systemic prednisolone during their pregnancy.
Methods
Patient population
We included all live-born infants whose mothers had used ≥10 mg/day of prednisolone (orally or intravenously) during ≥1 week prior to delivery, born in an 8-year period between October 2006 and October 2014 in the University Medical Center Groningen (UMCG). The UMCG is a tertiary hospital with about 1600 deliveries per year, and it is the only hospital in the region with a NICU. In the region covered by our hospital, all obstetricians and midwives must consult a neonatologist and pediatric endocrinologist if they treat a pregnant woman using systemic corticosteroids for a longer period of time and/or prior to delivery.
Data collection
Maternal and pediatric data were collected retrospectively from medical files.
Guideline procedures
The neonatologist is consulted for every live-born infant of mothers who used prednisolone prior to delivery. For mothers who used ≥10 mg/day of prednisolone, their newborn was admitted to the maternity ward. The child was admitted to the NICU for 48 h observation if the mother used ≥35 mg/day of prednisolone. All newborns were routinely assessed by physical examination and laboratory tests during their first 48 h. Dynamic testing of the HPA axis was considered only in cases of persistent findings of adrenal suppression. Newborns were followed up until 6 weeks after birth.
Definition of outcome measures
Blood pressure was measured (by full-automatic oscillometric measurement device), during the first 48 h of life and, after 48 h, when clinically indicated. Hypotension was defined as a mean blood pressure in mmHg below the tenth percentile for gestational and postnatal age.
Serum glucose level was measured by an enzymatic method (hexokinase-mediated reaction, Roche Modular, Mannheim, Germany) and assessed in all newborns, every 3 h for their first 48 h. Samples were taken before oral feedings. Hypoglycemia was defined as a blood glucose concentration <2.6 mmol/L (46 mg/dL).
Serum cortisol and plasma ACTH level was assessed in all newborns within the first 48 h. Ethylenediaminetetraacetic acid (EDTA) tubes were used to collect blood samples for ACTH, which were placed on ice and, after centrifugation, frozen immediately until analysis. The analysis was performed by electrochemiluminescence immunoassay using Cobas 6000 E601 (Roche Diagnostics, intra-assay coefficient of variation (CV) 0.6–3.6% and inter-assay CV 3.5–5.4%). Plasma cortisol was analyzed by electrochemiluminescence immunoassay using Modular E170 (Roche Diagnostics, inter-assay CV 2.3–4.0%). As newborns do not have a circadian rhythm of cortisol secretion, samples were taken at random moments during the day [4]. In screening adrenal dysfunction, a combined measurement of ACTH and cortisol in blood is used as standard practice at the UMCG. Adrenal dysfunction was defined if the ACTH level was >45.5 ng/L (10 pmol/L) and/or the cortisol level was <200 nmol/L in full-term newborns or 100 nmol/L in pre-term newborns. These cutoff levels have been used for many years as institutional reference ranges in clinical practice at the UMCG and are in accordance with the literature [7, 9].
Urinary steroid profile (USP) was assessed in all newborns in the first 48 h by taking a sample from a urine collection bag [2]. After enzymatic hydrolysis of urine samples, steroids were extracted and derivatized. Derivatized samples were analyzed using quantitative gas chromatography/mass spectrometry (GC/MS) with capillary chromatography. If fetal metabolites were absent, the USP was defined as abnormal and repeated after 2–4 weeks.
Results
Patient population
We enrolled 16 newborns in the study. Newborn and maternal characteristics are described in Table 1. Gestational age varied from 26 2/7 to 41 3/7 weeks (mean 34 5/7 ± 4 4/7 weeks) and birth weight varied from 885 to 4175 g (mean 2477 ± 1002 g). The dose and duration of prednisolone use varied from 10 to 60 mg/day (mean 29.7 ± 16.1 mg/day) and from one to 40 weeks (mean 18.4 ± 15.4 weeks) prior to delivery.
Outcome
Five newborns had transient hypoglycemia (Table 1). They all showed well-known risk factors for hypoglycemia (prematurity (n = 3), birth weight large for gestational age (n = 1), and maternal insulin-dependent diabetes gravidarum (n = 1)). They received extra oral feedings and/or intravenous glucose administration according to the UMCG guideline for neonatal hypoglycemia. All five newborns had normal serum cortisol levels and USP.
One newborn (patient 11, Table 1) showed a low serum cortisol level of 80 nmol/L. She did not have hypoglycemia or hypotension. The USP showed presence of fetal metabolites. Therefore, dynamic testing of the HPA axis was not performed.
In two newborns (patients 9 and 16, Table 1), USP from a urine sample collected on the first day of life showed the absence of fetal metabolites. Both infants were born pre-term, did not show hypoglycemia, but did have hypotension during an episode of clinical sepsis (patient 9 on the 15th day and patient 16 on the 5th day after birth). They were treated with a single intravenous fluid bolus with saline and broad-spectrum antibiotics. They did not receive steroid replacement therapy. Serum cortisol levels were in the normal range. Within 4 weeks, the USP of both children contained neonatal steroid hormone metabolites.
Discussion
In this retrospective analysis during a time period of 8 years, we found 16 live-born children of mothers using ≥10 mg/day of prednisolone for ≥1 week prior to delivery. The chronic use of high-dosed prednisolone during pregnancy is thus not of frequent occurrence. We found no clinical symptoms of adrenal suppression, only small abnormalities in the laboratory results of half of these newborns (eight out of 16) which we will describe in the following paragraphs.
Five newborns showed hypoglycemia. However, it seems unlikely that these cases were related to HPA axis suppression as all had a normal USP and normal serum cortisol levels. Furthermore, they all had known risk factors for hypoglycemia (e.g., prematurity, birth weight large for gestational age, and maternal insulin-dependent diabetes gravidarum) and would therefore have been detected through our local hospital guideline.
We found one newborn (patient 11) with a low serum cortisol level (80 nmol/L). As newborns do not have a circadian rhythm of cortisol secretion [4], this finding might have been the result of taking samples on random moments during the day. We did not find USP abnormalities or clinical symptoms suggesting HPA axis suppression in this newborn.
In two newborns (patients 9 and 16), the first USP lacked fetal metabolites although serum cortisol levels were normal. During an episode of clinical late-onset sepsis, both newborns developed hypotension, for which they received a single intravenous fluid bolus with saline and broad-spectrum antibiotics. They did not receive steroid replacement therapy. Within 4 weeks after birth, both had USPs containing neonatal steroid hormone metabolites. Their response to sepsis without needing steroid replacement therapy suggests that both these newborns had an adequate adrenal stress response despite a transient abnormal USP. We can thus conclude that both pre-terms showed transient abnormal USP without clinical symptoms of adrenal insufficiency.
Since both these newborns were pre-term (28 and 30 weeks of gestational age), the immaturity of their HPA axis might have affected the USP. Furthermore, the enzymatic activity of 11-beta-HSD2 in the placenta could have been decreased because of immaturity, resulting in a higher transplacental passage of prednisolone. Indeed, a positive association between gestational age and 11-beta-HSD2 activity was previously described [3].
In both cases, placental bed pathology was suspected (one mother had hemolysis with elevated liver enzymes and low platelets (HELLP) syndrome, and the other had pregnancy-induced hypertension (PIH) combined with intra-uterine growth restriction). No other cases of placental bed pathology were found in our database. Placental bed pathology might influence the function of 11-beta-HSD2, resulting in a higher (more than double) transplacental passage of prednisolone [5]. However, a more recent study found contrary evidence in women with HELLP syndrome [10], reporting prednisolone levels of about 10% of maternal levels in the umbilical cords, similar to the levels seen in women without HELLP syndrome [6].
Broad reference ranges used in newborn HPA testing may suggest lack of specificity. Serum cortisol can be affected by an intrinsic production failure (due to immaturity of the HPA axis), as well as extrinsic factors such as stress during and after delivery [7]. We therefore feel that the interpretation of normal serum cortisol levels should take into account the clinical circumstances and the presence or absence of clinical signs of adrenal insufficiency. Additional information on the adrenal function can be provided by USP. Dynamic function testing of the HPA axis (e.g., ACTH stimulation testing) can confirm adrenal insufficiency by measuring the adrenal response to stress, and thus provides the most relevant information for clinical practice. Because our newborns did not show persistent signs of adrenal suppression, dynamic testing of the HPA axis was not considered necessary.
Study limitations
Although this is the first case series report on the subject, the most important limitation of our study is the relatively small number of patients and its retrospective character. It is possible that the obstetricians and midwives may not have reported a mother using prednisolone during pregnancy and that we might thereby have missed a case. Yet, all newborns in our region who would have developed symptomatic adrenal insufficiency would have been admitted to our NICU.
We found a great heterogeneity in patient characteristics and a wide variation in the dosage of prednisolone and duration of treatment. Both the small number of patients and the heterogeneity in patient characteristics in our study make it difficult to exclude a possible effect of the use of maternal prednisolone on the neonatal HPA axis. This uncertainty mainly applies to the different gestational ages of the children. It could be hypothesized that the influence of maternal prednisolone use on the neonatal HPA axis is more pronounced when the gestational age of the infant is younger. A multicenter, prospective cohort study on a larger number of patients would allow analyses of sub-groups, e.g., full-term vs. pre-term born infants, effect of dosage, and duration of prednisolone treatment and would allow risk factors to be identified using logistic regression analysis. Such a cohort study would also benefit from dynamic testing of the HPA axis in newborns showing biochemical evidence of adrenal suppression, which would allow evaluation of the adrenal response to stress.
Conclusion
In this retrospective case series of 16 newborns, we found no evidence of prolonged effects on the neonatal HPA axis of mothers using prednisolone during pregnancy. Small and transient biochemical abnormalities were not associated to clinical symptoms of adrenal insufficiency. However, a prospective cohort study with a large number of newborns is needed to further elucidate the effect of maternal corticosteroid use on the neonatal HPA axis.
Abbreviations
- 11-beta-HSD2:
-
11-beta-hydroxysteroid dehydrogenase-2
- ACTH:
-
adrenocorticotropic hormone
- CV:
-
coefficient of variation
- EDTA:
-
ethylenediaminetetraacetic acid
- GC/MS:
-
gas chromatography/mass spectrometry
- HELLP:
-
hemolysis with elevated liver enzymes and low platelets
- HPA:
-
hypothalamic-pituitary-adrenal
- NICU:
-
neonatal intensive care unit
- PIH:
-
pregnancy-induced hypertension
- UMCG:
-
University Medical Center Groningen
- USP:
-
urinary steroid profile
References
Dodds HM, Taylor PJ, Johnson LP, Mortimer RH, Pond SM, Canell GR (1997) Cortisol metabolism and its inhibition by glycyrrhetinic acid in the isolated perfused human placental lobe. J Steroid Biochem Mol Biol 62:337–343
Heckmann M, Hartmann MF, Kampschulte B, Gack H, Bödeker RH, Gortner L, Wudy SA (2005) Assessing cortisol production in preterm infants: do not dispose of the nappies. Pediatr Res 57:412–418
Hofmann M, Pollow K, Bahlmann F, Casper F, Steiner E, Brockerhoff P (2001) 11ß-hydroxysteroid dehydrogenase (11ß-HSD-II) activity in human placenta: its relationship to placental weight and birth weight and its possible role in hypertension. J Perinat Med 29:23–30
Mantagos S, Moustogiannis A, Vagenakis AG (1998) Diurnal variation of plasma cortisol levels in infancy. J Pediatr Endocrinol Metab 11:549–553
McCalla CO, Nacharaju VL, Muneyyirci-Delale O, Glasgow S, Feldman JG (1998) Placental 11 beta-hydroxysteroid dehydrogenase activity in normotensive and pre-eclamptic pregnancies. Steroids 63:511–515
Murphy VE, Fittock RJ, Zarzycki PK, Delahunty MM, Smith R, Clifton VL (2007) Metabolism of synthetic steroids by the human placenta. Placenta 28:39–46
Ng PC, Lam CW, Lee CH, Ma KC, Fok TF, Chan IH, Wong E (2002) Reference ranges and factors affecting the human corticotrophin-releasing hormone test in preterm, very low birth weight infants. J Clin Endocrinol Metab 87:4621–4628
Saulnier PJ, Piguel X, Perault-Pochat MC, Csizmadia-Bremaud C, Saulnier JP (2010) Hypoglycaemic seizure and neonatal acute adrenal insufficiency after maternal exposure to prednisone during pregnancy: a case report. Eur J Pediatr 169:763–765
Soliman AT, Taman KH, Rizk MM, Nasr IS, Alrimawy H, Hamido MS (2004) Circulating adrenocorticotropic hormone (ACTH) and cortisol concentrations in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: cortisol response to low-dose and standard-dose ACTH tests. Metabolism 53:209–214
Van Runnard Heimel PJ, Schobben AF, Huisjes AJ, Franx A, Bruinse HW (2005) The transplacental passage of prednisolone in pregnancies complicated by early-onset HELLP syndrome. Placenta 26:842–845
Acknowledgments
We thank Prof. P.J.J. Sauer for his comments during the writing of this article.
Author information
Authors and Affiliations
Contributions
Drs. de Vetten carried out the analyses of the collected data, drafted the initial manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted.
Dr. van Stuijvenberg critically reviewed the manuscript and approved the final manuscript as submitted.
Prof. Dr. Kema critically reviewed the manuscript and approved the final manuscript as submitted.
Dr. Bocca coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
No potential conflict of interest exists with this research, and no sponsors were involved.
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration or comparable ethical standards.
The local medical ethics committee concluded that no individual informed consents were required in this case series, as it consists of an analysis of standard hospital care.
Additional information
Communicated by Patrick Van Reempts
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
de Vetten, L., van Stuijvenberg, M., Kema, I.P. et al. Maternal use of prednisolone is unlikely to be associated with neonatal adrenal suppression—a single-center study of 16 cases. Eur J Pediatr 176, 1131–1136 (2017). https://doi.org/10.1007/s00431-017-2949-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-2949-1