Abstract
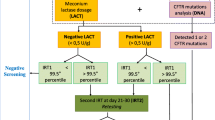
Cystic fibrosis (CF) is a life-threatening disease for which early diagnosis following newborn screening (NBS) improves the prognosis. We performed a prospective assessment of the immunoreactive trypsinogen (IRT)/DNA/IRT protocol currently in use nationwide, versus the IRT/pancreatitis-associated protein (PAP) and IRT/PAP/DNA CF NBS protocols. Dried blood spots (DBS) from 106,522 Czech newborns were examined for IRT concentrations. In the IRT/DNA/IRT protocol, DNA-testing was performed for IRT ≥ 65 ng/mL. Newborns with IRT ≥ 200 ng/mL and no detected cystic fibrosis transmembrane conductance regulator gene (CFTR) mutations were recalled for a repeat IRT. In the same group of newborns, for both parallel protocols, PAP was measured in DBS with IRT ≥ 50 ng/mL. In PAP-positive newborns (i.e., ≥1.8 if IRT 50–99.9 or ≥1.0 if IRT ≥ 100, all in ng/mL), DNA-testing followed as part of the IRT/PAP/DNA protocol. Newborns with at least one CFTR mutation in the IRT/DNA/IRT and IRT/PAP/DNA protocols; a positive PAP in IRT/PAP; or a high repeat IRT in IRT/DNA/IRT were referred for sweat testing. Conclusion: the combined results of the utilized protocols led to the detection of 21 CF patients, 19 of which were identified using the IRT/DNA/IRT protocol, 16 using IRT/PAP, and 15 using IRT/PAP/DNA. Decreased cut-offs for PAP within the IRT/PAP protocol would lead to higher sensitivity but would increase false positives. Within the IRT/PAP/DNA protocol, decreased PAP cut-offs would result in high sensitivity, an acceptable number of false positives, and would reduce the number of DNA analyses. Thus, we concluded that the IRT/PAP/DNA protocol would represent the most suitable protocol in our conditions.

Similar content being viewed by others
Abbreviations
- CF:
-
Cystic fibrosis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator gene
- DBS:
-
Dried blood spot
- DNA:
-
Deoxyribonucleic acid
- IRT:
-
Immunoreactive trypsinogen
- NBS:
-
Newborn screening
- PAP:
-
Pancreatitis-associated protein
- PSP:
-
Population-specific percentile
References
Baker MW, Groose M, Hoffman G, Rock M, Levy H, Farrell PM (2011) Optimal DNA tier for the IRT/DNA algorithm determined by CFTR mutation results over 14 years of newborn screening. J Cyst Fibros 10(4):278–81
Balascaková M, Holubová A, Skalická V, Zemková D, Kracmar P, Gonsorcíkova L, Camajová J, Piskácková T, Lebl J, Drevínek P, Gregor V, Vávrová V, Votava F, Macek M Jr (2009) Pilot newborn screening project for cystic fibrosis in the Czech Republic: defining role of the delay in its symptomatic diagnosis and influence of ultrasound-based prenatal diagnosis on the incidence of the disease. J Cyst Fibros 8(3):224–7
Bobadilla JL, Macek M Jr, Fine JP, Farrell PM (2002) Cystic fibrosis: a worldwide analysis of CFTR mutations-correlation with incidence data and application to screening. Hum Mutat 19(6):575–606
Brunecký Z (1972) The incidence and genetics of cystic fibrosis. J Med Genet 9(1):33–7
Castellani C, Cuppens H, Macek M Jr, Cassiman JJ, Kerem E, Durie P, Tullis E, Assael BM, Bombieri C, Brown A, Casals T, Claustres M, Cutting GR, Dequeker E, Dodge J, Doull I, Farrell P, Ferec C, Girodon E, Johannesson M, Kerem B, Knowles M, Munck A, Pignatti PF, Radojkovic D, Rizzotti P, Schwarz M, Stuhrmann M, Tzetis M, Zielenski J, Elborn JS (2008) Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 7(3):179–96
Castellani C, Southern KW, Brownlee K, Dankert Roelse J, Duff A, Farrell M, Mehta A, Munck A, Pollitt R, Sermet-Gaudelus I, Wilcken B, Ballmann M, Corbetta C, de Monestrol I, Farrell P, Feilcke M, Férec C, Gartner S, Gaskin K, Hammermann J, Kashirskaya N, Loeber G, Macek M Jr, Mehta G, Reiman A, Rizzotti P, Sammon A, Sands D, Smyth A, Sommerburg O, Torresani T, Travert G, Vernooij A, Elborn S (2009) European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros 8(3):153–73
Comeau AM, Parad RB, Dorkin HL, Dovey M, Gerstle R, Haver K, Lapey A, O'Sullivan BP, Waltz DA, Zwerdling RG, Eaton RB (2004) Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: a cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatr 113(6):1573–81
Comeau AM, Accurso FJ, White TB, Campbell PW 3rd, Hoffman G, Parad RB, Wilfond BS, Rosenfeld M, Sontag MK, Massie J, Farrell PM, O'Sullivan BP, Foundation CF (2007) Guidelines for implementation of cystic fibrosis newborn screening programs: cystic fibrosis foundation workshop report. Pediatr 119(2):e495–518
Crossley JR, Smith PA, Edgar BW, Gluckman PD, Elliott RB (1981) Neonatal screening for cystic fibrosis, using immunoreactive trypsin assay in dried blood spots. Clin Chim Acta 113(2):111–21
Dagorn JC (2011) International Society of Neonatal Screening [homepage on the Internet]. CF newborn screening with the MucoPAP kits: changing the dilution factor. Available from: http://www.isnsneoscreening.org/htm/news_detail.htm?id=130 updated 4th March 2011
Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, Hoffman G, Laessig RH, Splaingard ML (2001) Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatr 107(1):1–13
Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW 3rd, Cystic Fibrosis Foundation (2008) Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 153(2):S4–S14
Lai HJ, Cheng Y, Cho H, Kosorok MR, Farrell PM (2004) Association between initial disease presentation, lung disease outcomes, and survival in patients with cystic fibrosis. Am J Epidemiol 159(6):537–46
LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ Jr, Cystic Fibrosis Foundation (2007) Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr 151(1):85–9
Mayell SJ, Munck A, Craig JV, Sermet I, Brownlee KG, Schwarz MJ, Castellani C, Southern KW, European Cystic Fibrosis Society Neonatal Screening Working Group (2009) A European consensus for the evaluation and management of infants with an equivocal diagnosis following newborn screening for cystic fibrosis. J Cyst Fibros 8(1):71–8
Ross LF (2008) Newborn screening for cystic fibrosis: a lesson in public health disparities. J Pediatr 153(3):308–13
Sarles J, Barthellemy S, Férec C, Iovanna J, Roussey M, Farriaux JP, Toutain A, Berthelot J, Maurin N, Codet JP, Berthézène P, Dagorn JC (1999) Blood concentrations of pancreatitis-associated protein in neonates: relevance to neonatal screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed 80(2):F118–22
Sarles J, Berthézène P, Le Louarn C, Somma C, Perini JM, Catheline M, Mirallié S, Luzet K, Roussey M, Farriaux JP, Berthelot J, Dagorn JC (2005) Combining immunoreactive trypsinogen and pancreatitis-associated protein assays, a method of newborn screening for cystic fibrosis that avoids DNA analysis. J Pediatr 147(3):302–5
Scotet V, Assael BM, Duguépéroux I, Tamanini A, Audrézet MP, Férec C, Castellani C (2008) Time trends in birth incidence of cystic fibrosis in two European areas: data from newborn screening programs. J Pediatr 152(1):25–32
Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A (2007) Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatr 119(1):19–28
Sommerburg O, Lindner M, Muckenthaler M, Kohlmueller D, Leible S, Feneberg R, Kulozik AE, Mall MA, Hoffmann GF (2010) Initial evaluation of a biochemical cystic fibrosis newborn screening by sequential analysis of immunoreactive trypsinogen and pancreatitis-associated protein (IRT/PAP) as a strategy that does not involve DNA testing in a Northern European population. J Inherit Metab Dis 33(suppl2):S263–71
Sontag MK, Corey M, Hokanson JE, Marshall JA, Sommer SS, Zerbe GO, Accurso FJ (2006) Genetic and physiologic correlates of longitudinal immunoreactive trypsinogen decline in infants with cystic fibrosis identified through newborn screening. J Pediatr 149(5):650–67
Stopsack M (2011) Improved cut off combination for IRT and PAP in newborn screening for cystic fibrosis. J Cyst Fibros 10(suppl1):S6
Thauvin-Robinet C, Munck A, Huet F, Génin E, Bellis G, Gautier E, Audrézet MP, Férec C, Lalau G, Georges MD, Claustres M, Bienvenu T, Gérard B, Boisseau P, Cabet-Bey F, Feldmann D, Clavel C, Bieth E, Iron A, Simon-Bouy B, Costa C, Medina R, Leclerc J, Hubert D, Nové-Josserand R, Sermet-Gaudelus I, Rault G, Flori J, Leroy S, Wizla N, Bellon G, Haloun A, Perez-Martin S, d‘Acremont G, Corvol H, Clément A, Houssin E, Binquet C, Bonithon-Kopp C, Alberti-Boulmé C, Morris MA, Faivre L, Goossens M, Roussey M, Collaborating Working Group on R117H, Girodon E (2009) The very low penetrance of cystic fibrosis for the R117H mutation: a reappraisal for genetic counselling and newborn screening. J Med genet 46(11):752–8
Vernooij-van Langen AM, Loeber JG, Elvers B, Triepels RH, Gille JJ, Van der Ploeg CP, Reijntjens S, Dompeling E, Dankert-Roelse JE, on behalf of the CHOPIN Study Group (2012) Novel strategies in newborn screening for cystic fibrosis: a prospective controlled study. Thorax 67(4):289–295
Acknowledgments
This study was supported by a grant from the Czech Ministry of Health: IGA NS 9986-3/2008 to FV. We would like to acknowledge all collaborating primary care physicians and clinicians from neonatal units who we could not list individually. We would like to thank Mr Thomas Secrest for linguistic proofreading of the final manuscript. Finally, we would like to thank all participants of the parallel CF NBS research protocols.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Veronika Krulišová and Miroslava Balaščaková contributed equally to this paper. Senior authors, Felix Votava and Milan Macek Jr., also contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Krulišová, V., Balaščaková, M., Skalická, V. et al. Prospective and parallel assessments of cystic fibrosis newborn screening protocols in the Czech Republic: IRT/DNA/IRT versus IRT/PAP and IRT/PAP/DNA. Eur J Pediatr 171, 1223–1229 (2012). https://doi.org/10.1007/s00431-012-1747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-012-1747-z