Abstract
Neurological manifestations and outcomes of children with the 2009 H1N1 virus infection have been reported in three American series and from smaller cohorts and case reports worldwide. Of the 83 children admitted between April 2009 and March 2010 with H1N1 virus infection to a tertiary children’s hospital in a European setting, five children aged between 2 and 10 years had neurological symptoms. Four patients had seizures and encephalopathy at presentation. One patient presented with ataxia; one developed neuropsychiatric manifestations, and two developed movement disorders during the disease course. Early neuroimaging showed evidence of acute necrotising encephalopathy (ANE) in one case and non-specific white matter changes in another. Initial neuroimaging was normal for the other three, but interval MRI showed increased signal in bilateral periventricular distribution in one and significant cerebral volume loss in the other. Clinical outcomes varied: two recovered fully while three had residual seizures and/or significant cognitive deficits. Conclusion An analysis of our patients along with all reported cases reveal that seizures and encephalopathy were common neurological presentations associated with pandemic 2009 H1N1 influenza virus infection in children requiring hospital admission. Neuroimaging suggestive of ANE, basal ganglia involvement and volume loss appears to be associated with worse neurological outcome.
Similar content being viewed by others
Introduction
Neurological complications are well recognised in influenza infection, with different strains demonstrating varying degrees of neurovirulence [17, 34]. The “Spanish Flu” pandemic of 1918–1930, estimated to have infected 500 million people and claimed 40 million lives, caused major central nervous system morbidity [34]. More recently, the epidemic influenza A (H3N2) strain was associated with a surge in cases of encephalitis/encephalopathy in Japan [18, 28, 46], Europe [40] and America [25]. The spectrum of neurological conditions arising from neurovirulent strains of influenza includes seizures [4, 25, 40], acute necrotising encephalopathy (ANE) [30, 40, 42, 44], Reye’s syndrome [3], transverse myelitis [21], acute disseminated encephalomyelitis [21], Guillain–Barre syndrome [9, 21], mutism [29] and movement disorders [37].
The pandemic influenza A 2009 H1N1 virus spread worldwide claiming over 18,400 lives [35] with a notable predominance of disease burden and mortality in children [13, 32, 38]. In England, the paediatric mortality was estimated at six per million (70 cases) from an observational population-based study [38]. Thus far, neurological complications have been reported in 26 paediatric cases from three case series from America [2, 7, 8] and in 38 additional cases worldwide [1, 5, 11, 14, 16, 22–24, 26, 31–33, 36, 39, 41, 43, 45]. Table 1 details the neurological presentation and outcome of these paediatric cases. In this paper, we report the neurological manifestations and outcomes of children who presented to a single UK institution with the 2009 H1N1 virus infection and review their neurological complications in conjunction with the previously reported cases from this epidemic worldwide.
Methods
Patient selection and case definitions
The Evelina Children’s Hospital (ECH) admits children under the age of 16 years from the local population and is the regional referral centre for paediatric intensive care, neurology and infectious disease for South East London. ECH has 140 paediatric beds, admits 20,000 children annually and serves a population of seven million (1.5 million children). Hospital data (case notes) of all children hospitalised with the 2009 H1N1 virus infection identified from microbiological records between 1 April 2009 to 31 March 2010 were reviewed. A child with acute neurological complications associated with the 2009 H1N1 virus infection was defined as having laboratory-confirmed infection with seizures, encephalopathy, encephalitis or any focal neurological syndrome (e.g. ataxia) within 1 week of an influenza-like symptom. Children with pre-existing epilepsy who presented with seizures were excluded. Encephalopathy was defined as altered level of consciousness for more than 24 h, including lethargy, irritability or change in personality and behaviour [12]. Encephalitis was diagnosed when encephalopathy was present with two or more of: fever or history of fever (≥38°C), seizures and/or focal neurological findings (with evidence of brain parenchyma involvement), cerebrospinal fluid (CSF) pleocytosis (>4 white blood cells/μl), electroencephalogram (EEG) findings in keeping with encephalitis and neuroimaging in keeping with encephalitis [12].
2009 H1N1 virus infection was confirmed by detection of influenza viral ribonucleic acid (RNA) in upper respiratory tract (throat/nasal swabs) or lower respiratory tract (bronchoalveolar lavage fluid) specimens. Reverse transcriptase polymerase chain reaction was performed using primers and probes specific for the haemagglutinin gene of the 2009 H1N1 virus according to a method standardised by the Health Protection Agency of the United Kingdom [15]. Respiratory samples were also processed to detect concurrent infection with seasonal influenza A and B viruses, respiratory syncytial viruses A and B, parainfluenza viruses 1–4, adenovirus, human metapneumovirus, entero/rhinoviruses, human coronaviruses and bocavirus using a multiplex PCR method. Serological tests were performed to exclude recent infection with cytomegalovirus, Epstein-Barr virus, Mycoplasma pneumoniae and Toxoplasma gondii. CSF samples were investigated for herpes simplex virus (HSV) deoxyribonucleic acid (DNA), varicella–zoster virus DNA, enterovirus RNA and 2009 H1N1 virus RNA.
Outcomes were defined as previously described: level 1, normal; level 2, mild sequelae; level 3, severe sequelae requiring help with personal daily activities and level 4, death [28]. All patients have up to a minimum of 9 months follow-up period. This study was conducted as a retrospective note review. Consent was obtained from the five patients for the reporting of the case history.
Results
Eighty-three children were admitted with 2009 H1N1 virus infection, of which five had neurologic symptoms. Table 2 summarises the clinical characteristics, treatment and outcome, and Table 3 illustrates the investigation of the five patients.
Patient reports
Case 1
A 3-year-old Afro-Caribbean boy presented with three self-limiting febrile seizures following 3 days of fever and coryza. He developed generalised afebrile seizures and truncal ataxia on day 2. Brain magnetic resonance imaging (MRI) on that day showed parietal–occipital white matter changes. EEG showed 1–2 Hz slow wave activity with no epileptic discharges, and CSF analysis was normal. He was treated with intravenous (IV) acyclovir, IV cefotaxime and oral clarithromycin. Respiratory secretions tested positive for 2009 H1N1 virus RNA; he was commenced on oral oseltamivir. His ataxia improved, and at discharge (day 4), he was seizure-free. He was readmitted 8 days later having more seizures but remained neurologically normal. Repeat CSF analysis and brain MRI were unchanged. Seizure control was achieved by sodium valproate and phenytoin. One year later, he is seizure-free and off anticonvulsants but had significant delay in personal and social skills (outcome 2).
Case 2
A 4-year-old Caucasian girl presented with a 20-min generalised, tonic–clonic seizure following 2 days of fever and coryza. She was pyrexial (38.9°C) and had a herpetic lesion (HSV type 1 DNA-positive) on her upper lip. Seizures continued warranting sedation and ventilation. She was commenced on IV ceftriaxone, IV acyclovir and oral oseltamivir. 2009 H1N1 virus RNA was detected in upper and lower respiratory samples. CSF analyses were negative for H1N1 and HSV PCR. EEG on day 2 showed continuous generalised slow wave activity with no epileptiform discharges. She remained encephalopathic and developed upper motor neuron signs. Brain MRI on day 2 was suggestive of ANE. Due to the clinical and neuroimaging severity, she was given intravenous immunoglobulin (IVIG), and oral oseltamivir therapy was empirically converted to IV zanamivir. Her clinical recovery was excellent with neurological normalisation over 7 days. At 3 and 9 months, she remained neurologically normal and had fully integrated back in school (outcome 1).
Case 3
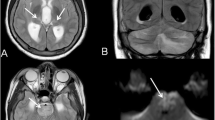
A 10-year-old Asian boy was admitted following a 10-min generalised seizure. He had a flu-like illness a week prior but had not been prescribed oseltamivir. He was drowsy but had no focal neurological signs. Brain computed tomography (CT) was normal, and CSF showed mild pleocytosis. He was treated with IV acyclovir, IV ceftriaxone, oral clarithromycin and oral oseltamivir. Upper respiratory tract sampling was positive for the 2009 H1N1 virus. Frequent seizures persisted, and EEG on day 5 showed high-amplitude generalised slow waves but no epileptiform discharges. Deterioration in seizure control necessitated sedation and ventilatory support. Brain MRI and repeat CSF analysis were normal. He received IVIG as an immune-mediated encephalopathy was suspected. Quadruple anticonvulsant therapy achieved sufficient seizure control to allow extubation after 10 days. Drowsiness, marked choreoathetoid movements, aphasia and motor weakness gradually improved, although seizure control was not sustained. Episodes of acute confusional state and aggression responded to risperidone therapy. He was discharged after 6 weeks, still having frequent focal and generalised seizures. He had two further courses of IVIG at monthly intervals. Repeat brain MRI at 3 months showed subtle increased signal in a periventricular distribution bilaterally. Neuro-metabolic investigations showed no evidence of an aminoacidaemia, organicacideamia, urea cycle or fatty oxidation defect. There was no identifiable evidence of an autoantibody-mediated encephalopathy. One year after the acute illness, his seizures remain refractory to treatment, necessitating admission to a specialised inpatient hospital educational setting. Personal care and learning are affected by significant neuropsychometric deficits in memory and executive functioning (outcome 3).
Case 4
A 5-year-old Caucasian girl presented with unabating seizures preceded by 2 days of cough, coryza and fever. Seizures were refractory to initial therapies and were ultimately terminated by thiopentone and midazolam infusion. She required ventilatory support, significant fluid resuscitation (50 ml/kg) for hypotensive shock as well as inotropic support for 48 h. She was commenced on IV ceftriaxone, IV acyclovir and oral oseltamivir. Her throat/nasal swab was positive for the 2009 H1N1 virus. Brain MRI scan on day 2 and CSF analysis on day 3 were normal. EEG on day 3 showed generalised 1–4 Hz slow waves with no epileptiform activity. Sustained seizure control was achieved after 4 days. She developed dyskinetic movements and dystonic posturing. There was little improvement following IVIG on day 6. EEG on day 12 was unchanged with no epileptiform activity corresponding with dyskinetic movements. Repeat MRI brain after 3 weeks showed significant cerebral volume loss and non-specific white matter changes in the right posterior parietal region. Neuro-metabolic investigations (as detailed for case 3) and autoantibody-mediated encephalopathy were negative. When reviewed at 4 months, seizures were well controlled, with resolution of her movement disorder, but at 11 months review, she exhibited significant cognitive impairment and social communication difficulties, requiring education in a special school (outcome 3).
Case 5
An 8-month-old Caucasian boy with Down’s syndrome had 7 days of cough and temperature prior to presenting with an unresponsiveness episode lasting for 10 min. He was encephalopathic and had mild respiratory distress requiring oxygen. Chest X-ray revealed bilateral peri-hilar consolidation. He was treated with IV ceftriaxone, IV acyclovir and oral clarithromycin for presumed meningo-encephalitis with pneumonia. After initial improvement in alertness, his level of consciousness reduced on day 3 associated with poor respiratory effort, necessitating ventilatory support. Respiratory secretions were positive for 2009 H1N1 virus, and oseltamivir was started. The remainder of the septic screen including CSF was negative. CT brain was normal. Following extubation, his neurological function was normal. He was discharged by day 8 and had fully recovered at 6 weeks, remaining neurologically well at 1 year review (outcome 1).
Discussion
The Evelina Children’s Hospital in London managed a large number of hospitalised H1N1 cases, including six of England’s 51 H1N1-associated in-hospital paediatric deaths [38]. Thirty percent (22/70) of the paediatric deaths in England presented with encephalopathy or seizures [38]. After death, neurological sequelae are amongst the most severe consequences of pandemic influenza A H1N1 infection in children [38]. Compared with children presenting with seasonal influenza, patients with 2009 H1N1 infections had more severe neurological complications (encephalopathy and focal neurology), whilst the incidence of seizures and status epilepticus were similar across both groups [7].
Neurological presentation and CSF investigation
The rate of neurological complications in children with 2009 H1N1 virus infection hospitalised to our institute was 6% (5/83) compared with 2% in the Pittsburg series [2], 7% in Utah [7], 10% in Turkey [45] and 15% in Texas [8]. Demographic data available for our cohort indicated that only one patient with neurological complication was from our local population (2%; 1/53) compared with the four patients referred from the region either via the intensive care unit or paediatric neurology service (13%, 4/30), highlighting the referral bias with referred patient tending to be more severely affected and having more neurological complications. Thus, the variation of incidence ranging from 2% to 15% from various studies is likely to reflect either the referral bias of the reporting institution or the inclusion of patients with underlying seizure disorder or neurological conditions.
Seizures and altered sensorium were the most common neurological symptoms, occurring in four of five patients in our series. Four of the five patients presented with encephalopathy, of which two met the criteria for encephalitis. Three of our patients required intensive care support for control of seizures and one for encephalopathy. Such severity of neurological complications associated with 2009 H1N1 influenza virus accords with that reported in the largest American series where ten out of 18 patients required intensive care admission [7]. Two of our patients developed both seizures and movement disorders; one developed neuropsychiatric manifestation during the disease evolution and is only the second reported case of this complication associated with the 2009 H1N1 pandemic strain [11]. Movement disorders following viral encephalitis have generally been associated with basal ganglia involvement [20, 21, 37]. Interestingly, while two of the cases reported here developed a movement disorder in the convalescent phase, neither had evidence of basal ganglia involvement on neuroimaging. The abnormal movements improved in both cases at follow-up.
Inclusive of our five cases, 62% (37/59; data not available in ten) of all reported cases of 2009 H1N1 with related neurological complications had seizures at presentation while 69% (41/59) had features of encephalopathy (Table 1). Thirteen percent (8/59) of patients had focal neurological signs at presentation. The novel H1N1 strain has been detected in CSF in only one case to date, although that result was possibly attributable to traumatic lumbar puncture [39], a finding similar to influenza associated encephalopathy (IAE) induced by other strains of influenza where CSF viral isolation has been a rarity [10, 46]. In our series, CSF pleocytosis was only seen in one case (case 3), comparable to the 18% (7/38) reported in the literature.
Neuroimaging
Of the 69 reported cases, brain neuroimaging data was available in 42. Neuroimaging was normal in 42% (18/42) of patients. Cortical and sub-cortical white matter signal changes (16%; 7/42) and neuroimaging features of ANE (17%; 7/42) are the commonest neuroimaging changes described. The importance of acute and follow-up neuroimaging is underlined by our series. Acutely, only two of our five cases had abnormal neuroimaging: one showed evidence of ANE and another showed non-specific white matter changes. Although initial neuroimaging was normal for the other three patients, the interval MRI was abnormal in two. One showed increased signal in periventricular distribution bilaterally, the other revealed significant loss of cerebral volume. Normal acute imaging followed by brain atrophy is well described with IAE, most patients being left with marked neurological sequelae [2, 46], as in our patient. Patients infected with the 2009 H1N1 strain with ANE, basal ganglia involvement and volume loss on neuroimaging had poorer outcome (Table 1).
Treatment
All five cases in this series were treated with oseltamivir (Roche, Welwyn Garden City, England). Case 2 was empirically changed to IV zanamivir due to the severity of this child’s clinical condition and the potential severity of outcome associated with ANE and because of the potential of oseltamivir resistant virus. IV zanamivir was supplied on a compassionate basis by Glaxo-Smith-Kline (Brentford, England) and is thought to carry the same efficacy as oseltamivir [19]. Although the use of antiviral medications can decrease the risk of complications from influenza [6, 27], specific neuro-therapeutic effect is uncertain [25]. In line with previous reports, it was not possible to determine whether antiviral treatment decreased the severity or improved neurological outcome in this series or in the other reported cases.
IVIG was given in three patients for potentially immunomodulatory effects, indicated by the severity of presentation. While the timing of IVIG administration may influence its likelihood of conferring benefit, this series is too small to allow any inference to be drawn. There was little evidence of benefit in two patients. The third patient, with ANE, made complete recovery (case 2). This clinical outcome is unusual when compared with reported cases [2, 23, 24, 26, 33, 45] but cannot be attributed to any individual therapeutic intervention given the complex multi-therapeutic interventions the child received concurrently.
Outcome
In the Japanese study involving influenza A and B strains, elevated serum aspartate aminotransferase and creatine phosphokinase (CPK), and thrombocytopenia (<50,000 platelets/μl) appeared to correlate with unfavourable outcome in influenza-associated encephalopathy [28]. None of our cases had liver function abnormalities or thrombocytopenia; two had elevated CPK of more than 600 IU/L, both of which had poorer outcome. The cases presented in this series had varied outcomes. Two children (cases 2 and 5) recovered fully. Three patients developed seizure disorders; at 1 year, two have ongoing seizures (cases 3 and 4), one of which remains intractable to treatment. Three patients were left with mild (case 1) to severe (cases 3 and 4) cognitive impairment.
Out of the four deaths with neurological complications thus far reported, three were due to ANE [23, 26, 45] and one was due to associated respiratory failure [7]. Twelve of the 57 cases (18%; data not available in 12) required treatment with anti-epileptic medication and 12% (7/57) had significant neurological sequelae (Table 1). Although 65% (34/52) of patients were reported to have full recovery at discharge, longer-term follow-up of cases may indeed reveal additional cognitive impairment. Conversely, neurologically abnormal patients at discharge may indeed improve over time [2, 7]. Only longer-term follow-up will precisely determine the neurological burden of 2009 H1N1 influenza virus.
Conclusion
Seizures and encephalopathy are the common neurological phenotype associated with pandemic 2009 H1N1 influenza virus infection in children. While we await the emerging epidemiology of H1N1 and other strains of influenza in coming seasons, early consideration of influenza strains as a cause when children present with neurologic symptoms is advocated, given the potential for significant long-term sequelae especially in previously healthy children (57% of reported cases; 34/59). Normal neuroimaging at presentation should not be over-interpreted; interval imaging is advocated if recovery is not swift. Neuroimaging suggestive of ANE, basal ganglia involvement and volume loss appears to be associated with worse neurological outcome. Whether early treatment with antiviral drugs improves neurological outcome is unknown, but until there is evidence to guide management, early antiviral therapy is advocated. The role of immunomodulatory adjuvant therapy, however, remains speculative and likely should be reserved for the most severe cases.
References
Acevedo KLM, Ponce S, Cabezas A (2010) Influenza A H1N1 encephalitis: case report [abstract]. Dev Med Child Neurol 52(Suppl 1):P32
Baltagi SA, Shoykhet M, Felmet K et al (2010) Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med 11(2):179–184
Belay ED, Bresee JS, Holman RC et al (1999) Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med 340(18):1377–1382
Chiu SS, Tse CY, Lau YL et al (2001) Influenza A infection is an important cause of febrile seizures. Pediatrics 108(4):E63
Choi SY, Jang SH, Kim JO et al (2010) Novel swine-origin influenza A (H1N1) viral encephalitis. Yonsei Med J 51(2):291–292
Cooper NJ, Sutton AJ, Abrams KR et al (2003) Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 326(7401):1235
Ekstrand JJ, Herbener A, Rawlings J et al (2010) Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol Sep 23 doi:10.1002/ana.22184
Evans AS, Agadi S, Siegel SD et al (2009) Neuroloigcal complications associated with novel influenza A (H1N1) virus in children—Dallas, Texas, May 2009. MMWR: Morbidity and Mortality weekly. 24 July 58(28):773–778
Flewett TH, Hoult J (1958) Influenzal encephalopathy and postinfluenzal encephalitis. Lancet 2(7036):11–15
Fujimoto S, Kobayashi M, Uemura O et al (1998) PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet 352(9131):873–875
German-Diaz M, Pavo-Garcia R, Diaz-Diaz J et al (2010) Adolescent with neuropsychiatric symptoms associated with novel influenza A (H1N1) virus infection. Pediatr Infect Dis J 29(6):570–571
Glaser CA, Gilliam S, Schnurr D et al (2003) In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis 36(6):731–742
Hackett S, Hill L, Patel J et al (2009) Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet 374(9690):605
Haktanir A (2010) MR imaging in novel influenza A(H1N1)-associated meningoencephalitis. AJNR Am J Neuroradiol 31(3):394–395
HPA (2009) Swine-Lineage influenza A H1 specific fast real time PCR. National standard methods. Virology VSOP 29(2): Available at: http://www.hpa-standardmethods.org.uk/pdfsops.asp. Accessed on: July 2010)
Iwata A, Matsubara K, Nigami H et al (2010) Reversible splenial lesion associated with novel influenza A (H1N1) viral infection. Pediatr Neurol 42:447–450
Jelliffe S (1918) Nervous and mental disturbances of Influenza. N Y Med J 108:725–8, 55–57, 807–11
Kasai T, Togashi T, Morishima T (2000) Encephalopathy associated with influenza epidemics. Lancet 355(9214):1558–1559
Kawai N, Ikematsu H, Iwaki N et al (2008) A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect 56(1):51–57
Kullnat MW, Morse RP (2008) Choreoathetosis after herpes simplex encephalitis with basal ganglia involvement on MRI. Pediatrics 121(4):e1003–e1007
Leigh AD (1946) Infections of the nervous system occurring during an epidemic of influenza B. Br Med J 2(4485):936–938
Lister P, Reynolds F, Parslow R et al (2009) Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet 374(9690):605–607
Lyon JB, Remigio C, Milligan T et al (2010) Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol 40(2):200–205
Mariotti P, Iorio R, Frisullo G et al (2010) Acute necrotizing encephalopathy during novel influenza A (H1N1) virua infection. Ann Neurol 68:111–114
Maricich SM, Neul JL, Lotze TE et al (2004) Neurologic complications associated with influenza A in children during the 2003–2004 influenza season in Houston, Texas. Pediatrics 114(5):e626–e633
Martin A, Reade EP (2010) Acute necrotizing encephalopathy progressing to brain death in a pediatric patient with novel influenza A (H1N1) infection. Clin Infect Dis 50(8):e50–e52
Matheson NJ, Harnden AR, Perera R et al (2007) Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev (1):CD002744
Morishima T, Togashi T, Yokota S et al (2002) Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 35(5):512–517
Newland JG, Romero JR, Varman M et al (2003) Encephalitis associated with influenza B virus infection in 2 children and a review of the literature. Clin Infect Dis 36(7):e87–e95
Okumura A, Abe S, Kidokoro H et al (2009) Acute necrotizing encephalopathy: a comparison between influenza and non-influenza cases. Microbiol Immunol 53(5):277–280
O’Leary MF, Chappell JD, Stratton CW et al (2010) Complex febrile seizures followed by complete recovery in an infant with high-titre 2009 pandemic influenza A (H1N1) virus infection. J Clin Microbiol 48(10):3803–3805
O’Riordan S, Barton M, Yau Y et al (2010) Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ 182(1):39–44
Ormitti F, Ventura E, Summa A et al (2010) Acute necrotizing encephalopathy in a child during the 2009 influenza A (H1N1) pandemia: MR imaging in diagnosis and follow-up. AJNR Am J Neuroradiol 31(3):396–400
Oxford JS (2000) Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol 10(2):119–133
Pandemic (H1N1) 2009 - update 112, World Heatlh Organisation. http://www.who.int/csr/don/2010_08_06/en/index.html (accessed Aug 2010)
Rellosa N, Bloch KC, Shane AL et al (2010) Neurologic manifestations of pediatric novel H1N1 influenza infection. Pediatr Infect Dis J. Aug 31. [Epub ahead of print] doi: 10.1097/INF.0b013e3181f2de6f
Ryan MM, Procopis PG, Ouvrier RA (1999) Influenza A encephalitis with movement disorder. Pediatr Neurol 21(3):669–673
Sachedina N, Donaldson LJ (2010) Paediatric mortality related to pandemic influenza A H1N1 infection in England:an observational population-base study. Lancet. Published online 27th October 2010. doi:10.1016/S0140-6736(10)61195-6
Sánchez-Torrent L, Triviño-Rodriguez M, Suero-Toledano P et al (2010) Novel influenza A (H1N1) encephalitis in a 3-month-old infant. Infection 38(3):227–229
Steininger C, Popow-Kraupp T, Laferl H et al (2003) Acute encephalopathy associated with influenza A virus infection. Clin Infect Dis 36(5):567–574
Tan K, Prerna A, Leo YS (2010) Surveillance of H1N1-related neurological complications. Lancet Neurol 9(2):142–143
Troedson C, Gill D, Dale RC (2008) Emergence of acute necrotising encephalopathy in Australia. J Paediatr Child Health 44(10):599–601
Webster RI, Hazelton B, Suleiman J et al (2010) Severe encephalopathy with swine origin influenza A H1N1 infection in childhood: case reports. Neurology 74(13):1077–1078
Wong AM, Simon EM, Zimmerman RA et al (2006) Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol 27(9):1919–1923
Yildizdas D, Kendirli T, Arslankoylu AE et al (2010) Neurological complications of pandemic influenza (H1N1) in children. Eur J Pediatr Nov 26 [Epub ahead of print] doi:10.1007/s00431-010-1352-y
Yoshikawa H, Yamazaki S, Watanabe T et al (2001) Study of influenza-associated encephalitis/encephalopathy in children during the 1997 to 2001 influenza seasons. J Child Neurol 16(12):885–890
Acknowledgements
We are thankful to Dr. Keith Pohl, Dr. Elaine Hughes, Dr. Jean-Pierre Lin, Dr. Nuria Martinez-Alier, Dr. Claire Lundy, Dr. Tammy Hedderly, Dr. John Jackman, Dr. Artemis Gika and Dr. Karine Lascelles who were all closely involved in the care and management of these patients and for their helpful comments on this manuscript. We would also like to thank Dr. Atta Siddiqui for reviewing all the neuroimaging; Dr. Sushma Goyal for reviewing the electroencephalograms; Dr. Eithne MacMahon and Dr. Emma Aarons for assistance with virology; and Dr. Ruth Ackers for help with the dosing of zanamivir.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Pinki Surana and Shan Tang contributed equally to this article.
Rights and permissions
About this article
Cite this article
Surana, P., Tang, S., McDougall, M. et al. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr 170, 1007–1015 (2011). https://doi.org/10.1007/s00431-010-1392-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-010-1392-3