Abstract
The objective of this study was to evaluate clinical symptoms and findings on cranial ultrasound (CUS) in preterm infants with cerebellar haemorrhage through retrospective analysis of all preterm infants with a postnatal CUS or MRI diagnosis of cerebellar haemorrhage admitted in a tertiary care centre between January 2002 and June 2009. Fifteen infants were identified; median gestational age was 25 2/7 weeks and median birth weight 730 g. We discerned six types of haemorrhage: subarachnoid (n = 3), folial (n = 1), lobar (n = 9, of which 4 bilateral), giant lobar (n = 1, including vermis) and contusional (n = 1). Especially in infants with lobar cerebellar haemorrhage, CUS showed preceding or concurrent lateral ventricle dilatation, mostly without intraventricular haemorrhage (IVH). Thirteen infants suffered from notable, otherwise unexplained motor agitation in the days preceding the diagnosis. In conclusion, motor agitation may be a presenting symptom of cerebellar haemorrhage in preterm infants. Unexplained ventriculomegaly can be a first sign of cerebellar haemorrhage and should instigate sonographic exploration of the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebellar haemorrhage in preterm infants has become a focus of attention, as it is associated with neurodevelopmental sequelae and mortality [1, 11, 15, 17]. Prevalences ranging from 2.3% to 19% for very low birth weight infants (VLBW) have been reported [10, 15, 17, 21]. With regard to neurodevelopmental sequelae, cerebellar haemorrhage may play a role in the cognitive, learning and behavioural dysfunctions known to affect survivors of preterm birth [11]. To our knowledge, there are no descriptions of the clinical presentation of this lesion in the early neonatal period. Early recognition of large cerebellar lesions is possible by cranial ultrasound (CUS) through the anterior fontanelle. It is thought, however, that a combination of striking clinical information and targeted scanning through mastoid or posterolateral fontanelle should facilitate recognition, even of small lesions [5, 10, 15, 16, 21].
The aim of this study was to retrospectively analyse the clinical symptoms and CUS findings of preterm infants with cerebellar haemorrhage in order to increase understanding of presenting signs and symptoms.
Patients and methods
From a detailed database we identified all preterm infants (<37 weeks postmenstrual age) admitted to the Erasmus MC-Sophia Children's Hospital from January 2002 through June 2009 with a postnatal CUS or MRI diagnosis of cerebellar haemorrhage. Infants with chromosomal abnormalities and congenital brain anomalies were excluded. The enrolled infants had been screened by local protocol, which entailed at least two ultrasound studies in the first week of life, followed by weekly ultrasound studies until discharge. Standard imaging was performed through the anterior fontanelle. However, if a lesion in the posterior fossa was suspected, additional imaging was performed at 12–13 MHz through the mastoid fontanelle, also known as the posterolateral fontanelle [8]. Sonograms were obtained using a Sequoia system (Siemens, Mountain View, California), and with Esaote system (Mylab 70, Genova Italy). The imaging studies were performed by neonatologists with experience in neonatal US brain imaging. Cerebellar haemorrhage was defined as a unilateral or bilateral hyperechoic change in the cerebellar hemisphere(s), vermis or subarachnoid layer. Following vaginal breech delivery, haemorrhagic changes in both inferior cerebellar areas were interpreted as contusion due to occipital osteodiastasis. Supratentorial IVH was graded using Papile's criteria [19]. As CUS imaging was implicit to standard care, explicit parental consent was not requested.
Additional MRI had been performed, whenever feasible, if CUS findings suggested cerebellar haemorrhage. Scanning was performed on a 1.5-T GE EchoSpeed scanner (GE Medical Systems, Milwaukee, Wisconsin), using an MR-compatible incubator provided with a specialised high-sensitivity neonatal head coil. All CUS and MRI studies were independently reviewed by two neonatologists (JD, PG), who reached consensus on the diagnosis.
Demographic, perinatal, postnatal and short-term follow-up data were retrieved from the medical records. Perinatal data included delivery, gestational age, gender, birth weight, Apgar score and cord pH. Postnatal data included respiratory support, use of inotrope agents, patent ductus arteriosus (PDA), sepsis and thrombocytopenia. PDA was diagnosed by echocardiography. Clinical symptoms and CUS findings preceding the radiologic diagnosis of cerebellar haemorrhage were reviewed. Ventriculomegaly was defined as a ventricular index more than two standard deviations above Levene's mean ventricular index [9]. The different types of cerebellar haemorrhages found were templated.
Results
We identified 15 preterm infants with a postnatal diagnosis of cerebellar haemorrhage in the period from January 2002 through June 2009, 0.5% of all 3,201 preterm infants admitted to the NICU during this period and 1% of the VLBW infants born in this period. Perinatal details are presented in Table 1.
Median gestational age was 25 2/7 weeks (range, 24 6/7 to 32 1/7 weeks), and median birth weight was 730 g (range, 535 to 1,905 g). Nine infants were small for gestational age, eleven were male, and seven were twins. Three mothers were diagnosed with pre-eclampsia/HELLP syndrome; three presented with preterm premature rupture of membranes, and three presented with placental abruption. Eleven mothers received antenatal betamethasone.
Five infants were born by caesarean section because they showed cardiotocographic abnormalities indicating foetal distress. Four were born following vaginal breech delivery. None were asphyxiated at birth. All required endotracheal ventilation, and ten received inotropic agents. Four were mildly thrombocytopenic on the first day of life (range, 71,000–128,000/μL). Three infants with CUS diagnosis of cerebellar haemorrhage had become thrombocytopenic over the preceding days (range, 6,000–26,000/μL). Eleven patients were treated for PDA. All of these were initially treated with indomethacin. Six underwent surgical ligation after treatment with indomethacin was not successful.
The initial CUS scans in 12 infants were normal prior to detection of cerebellar haemorrhage. The initial cranial sonograms of the remaining three revealed periventricular leukomalacia (infant 3), left-sided subependymal haemorrhage with lateral ventricle dilatation and left cerebellar haemorrhage (infant 11), and right-sided subependymal haemorrhage (infant 12). In 11 infants, CUS showed lateral ventricle dilatation preceding identification or coincident with cerebellar haemorrhage, which on standard CUS could not be explained by supratentorial IVH. Lateral ventricle dilatation was transient in all cases; none required intervention.
In 12 infants, cerebellar haemorrhage was first identified with CUS, between days 2 and 23 of life (median, 9). In six of these, subsequent MRI confirmed the diagnosis. Two patients died before MRI could be obtained (infants 13–14). In three others, cerebellar haemorrhage was first identified on MRI, which had been obtained because of the following findings on CUS: extensive periventricular leukomalacia (infant 3), grade III IVH with venous infarction (infant 4) and hyperechoic globi pallidi (infant 8).
Thirteen infants presented with striking motor agitation—as independently reported and noted in the records by at least two nurses—2 to 5 days (median, 3) preceding the diagnosis of cerebellar haemorrhage. Jerking of the extremities had been noted as a specific sign in three of those. Nine of these 13 infants received analgesics because of agitation, and one received a sedative. Generally, morphine was used for analgesia. No NSAIDs were used for analgesia. None of the infants were diagnosed with seizures, and none were treated as such.
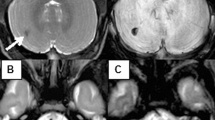
An overview of radiological and clinical findings is provided in Table 2 and Fig. 1. Figure 2 provides a CUS image of one of the included patients.
Discussion
We reviewed both clinical symptoms and CUS findings in 15 preterm infants with cerebellar haemorrhage. All but two had shown otherwise unexplained motor agitation over the days preceding the diagnosis, suggesting this may be a presenting symptom of cerebellar haemorrhage. CUS preceding or coincident with identification of cerebellar haemorrhage revealed lateral ventricle dilatation in most infants with lobar haemorrhage, which could not be explained by supratentorial IVH. To our knowledge, these findings have not previously been described in preterm infants.
Cerebellar haemorrhage in preterm infants was first described as a post-mortem finding in autopsy studies and in case reports [3, 20].
It is thought to be a multifactorial condition, with low gestational age and birth weight, vaginal delivery and instrumental or traumatic delivery as possible risk factors [4, 12, 15, 23]. The majority of cerebellar haemorrhages in this cohort were diagnosed in extremely preterm infants; 11 of 15 patients were born after less than 26 weeks of gestation. Four patients in this cohort were born following vaginal breech delivery. Mechanisms underlying cerebellar haemorrhage are poorly understood. Some authors suggest that it follows bleeding from the germinal matrix in the subependymal layer of the fourth ventricle roof or in the subpial external granule cell layer [2, 15]. One infant in the present study, who showed bilateral haemorrhagic changes in both lower hemispheres, probably suffered from occipital osteodiastasis associated with vaginal breech delivery [7]. Cerebellar haemorrhage may also develop after coagulopathy or an increase in venous pressure, for example, during extracorporeal membrane oxygenation [15, 18, 23]. Furthermore, emergency caesarean section, PDA and early acidosis may have an independent impact on the risk of cerebellar haemorrhage in preterm infants [10]. One third of the infants in the present study were indeed born after emergency caesarean section, and more than two thirds were diagnosed with PDA. The latter figure is slightly lower than the 80% reported by Limperopoulos et al. [10]. Three infants in our cohort were diagnosed with lobar cerebellar haemorrhage following supratentorial IVH. Cerebellar haemorrhage has been reported to be associated with ultrasonographically detectable supratentorial lesions, mostly IVH [22].
Until today, it was thought that preterm cerebellar haemorrhage remains clinically silent except for apnoea [14]. Based on our findings, we suggest, however, that otherwise unexplained motor agitation may be a symptom of cerebellar haemorrhage. The underlying mechanism could be explained as follows. The cerebellar cortex continuously inhibits signals sent to the brainstem and the thalamus by way of the efferent deep cerebellar nuclei. Destruction of cerebellar cortex, as in the case of cerebellar haemorrhage, will reduce the cortex's inhibitory capacity and thus lead to increased motor activity culminating in striking agitation [6]. In addition, pain due to meningeal haemorrhage may contribute to agitation.
Eleven infants in the present study, most with lobar haemorrhage, showed noteworthy ventricular dilatation on CUS, unexplained by IVH, prior to or coincident with discovery of cerebellar haemorrhage. Merill et al. described transient mild ventriculomegaly in one infant with cerebellar haemorrhage, but timing was not detailed [15]. We conclude that the ventricular dilatation in this study is a consequence of the cerebellar haemorrhage. Large cerebellar haemorrhages can impede the liquor circulation in several ways: by compressing the fourth ventricle as a result of the swelling in the acute stage of haemorrhage, by rupture of haemorrhage into the fourth ventricle, or by subarachnoid bleeding in the cisterna magna or underneath the tentorium. Our findings suggest that ventriculomegaly in the absence of any supratentorial intracranial haemorrhage may be a sign of cerebellar haemorrhage, especially in infants with lobar cerebellar haemorrhage. Unexplained ventriculomegaly is a reason to carefully explore both cerebellar hemispheres for lobar bleeding.
The incidence of cerebellar haemorrhage in our study period (0.5%) was much lower than in previous studies [1, 4, 10, 15, 17]. A possible explanation for this could be that we included infants less than 37 weeks gestation instead of only very low birth weight infants. Also, we may have missed cerebellar haemorrhages as ultrasound imaging through the mastoid fontanelle was not routine used. CUS through the mastoid fontanelle can detect cerebellar injury missed by using the anterior fontanelle approach [5, 13, 15, 21]. Posterior fossa views are generally not included, however, in standard CUS examination of preterm infants [21]. We recommend using posterior fossa views, especially when preterm infants show either unexplained ventricular dilatation or motor agitation.
Our report has limitations inherent to its retrospective design. Reporting of motor agitation was not structured, and preterm infants may have other concurrent diagnoses that could result in motor agitation. The findings of motoric unrest and ventriculomegaly in this study can now be studied prospectively in future studies.
Conclusions
In the described cohort, we found otherwise unexplained motor agitation in preterm infants to be a presenting symptom of cerebellar haemorrhage. Furthermore, unexplained ventricular dilatation hinted towards the lesion in infants with large cerebellar lesions.
References
Bednarek N, Akhavi A, Pietrement C et al (2008) Outcome of cerebellar injury in very low birth-weight infants: six case reports. J Child Neurol 23:906–911
Castillo M (2007) Selective vulnerability and the cerebellum in neonates. AJNR Am J Neuroradiol 28:20–21
Donat JF, Okazaki H, Kleinberg F (1979) Cerebellar hemorrhages in newborn infants. Am J Dis Child 133:441
Dyet LE, Kennea N, Counsell SJ et al (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Enriquez G, Correa F, Celestino A et al (2006) Mastiod fontanelle approach for sonographic imaging of the neonatal brain. Pediatr Radiol 36:532–540
Haines DE (2006) Fundamental neuroscience for basic and clinical applications, 3rd edn. Churchill Livingstone, Oxford
Hemsath FA (1934) Birth injury of the occipital bone with a report of thirty-two cases. Am J Obstet Gynecol 27:194–203
Korsten A, Lequin M, Govaert P (2006) Sonographic maturation of third-trimester cerebellar foliation after birth. Pediatr Res 59:695–699
Levene MI (1981) Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56:900–914
Limperopoulos C, Benson CB, Bassan H et al (2005) Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 116:717–724
Limperopoulos C, Bassan H, Gauvreau K et al (2007) Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120:584–593
Limperopoulos C, Robertson RL, Sullivan NR et al (2009) Cerebellar injury in term infants: clinical characteristics, magnetic resonance imaging findings, and outcome. Pediatr Neurol 41:1–8
Luna JA, Goldstein RB (2000) Sonographic visualization of neonatal posterior fossa abnormalities through the posterolateral fontanelle. AJR 174:561–567
Martin R, Roessmann U, Fanaroff A (1976) Massive intracerebellar hemorrhage in low-birth-weight infrants. J Pediatr 89:290–293
Merill JD, Piecuch RE, Fell SC et al (1998) A new pattern of cerebellar hemorrhages in preterm infants. Pediatrics 102:E62
Miall LS, Cornette LG, Tanner SF et al (2003) Posterior fossa abnormalities seen on magnetic resonance brain imaging in a cohort of newborn infants. J Perinatol 23:396–403
Müller H, Beedgen B, Schenk J et al (2007) Intracerebellar hemorrhage in premature infants: sonographic detection and outcome. J Perinat Med 35:67–70
Pape KE, Armstrong DL, Fitzhardinge PM (1976) Central nervous system pathology associated with mask ventilation in the very low birthweight infant: a new etiology for intracerebellar hemorrhages. Pediatrics 58:473–483
Papile LA, Burstein J, Burstein R et al (1978) Incidence and evolution of supependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 g. J Pediatr 92:529–534
Schreiber MS (1963) Posterior fossa (cerebellar) haematoma in the new-born. Med J Aust 26:713–715
Steggerda SJ, Leijser LM, Wiggers-de Bruïne FT et al (2009) Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology 252:190–199
Volpe JJ (2009) Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 24:1085–1104
Wigglesworth JS, Pape KE (1980) Pathophysiology of intracranial haemorrhage in the newborn. J Perinat Med 8:119–133
Competing interests
None declared.
Funding
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ecury-Goossen, G.M., Dudink, J., Lequin, M. et al. The clinical presentation of preterm cerebellar haemorrhage. Eur J Pediatr 169, 1249–1253 (2010). https://doi.org/10.1007/s00431-010-1217-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-010-1217-4