Abstract
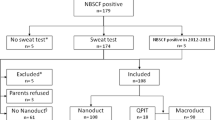
Determination of chloride concentration in sweat is the current diagnostic gold standard for Cystic Fibrosis (CF). Nanoduct® is a new analyzing system measuring conductivity which requires only 3 microliters of sweat and gives results within 30 minutes. The aim of the study was to evaluate the applicability of this system in a clinical setting of three children’s hospitals and borderline results were compared with sweat chloride concentration. Over 3 years, 1,041 subjects were tested and in 946 diagnostic results were obtained. In 95 children, Nanoduct® failed (9.1% failure rate), mainly due to failures in preterm babies and newborns. Assuming 59 mmol/L as an upper limit of normal conductivity, all our 46 CF patients were correctly diagnosed (sensitivity 100%, 95% CI: 93.1–100; negative predicted value 100% (95% CI: 99.6–100) and only 39 non CF’s were false positive (39/900, 4.3%; specificity 95.7%, 95%CI: 94.2–96.9, positive predicted value 54.1% with a 95%CI: 43.4–65.0). Increasing the diagnostic limit to 80 mmol/L, the rate fell to 0.3% (3/900). CF patients had a median conductivity of 115 mmol/L; the non-CF a median of 37 mmol/L. In conclusion, the Nanoduct® test is a reliable diagnostic tool for CF diagnosis: It has a failure rate comparable to other sweat tests and can be used as a simple bedside test for fast and reliable exclusion, diagnosis or suspicion of CF. In cases with borderline conductivity (60–80 mmol/L) other additional methods (determination of chloride and genotyping) are indicated.



Similar content being viewed by others
Abbreviations
- CF:
-
Cystic fibrosis
- CI:
-
Confidence interval
- DC:
-
Direct current
- GA:
-
Gestational age
- IRT:
-
Immune reactive trypsinogen
- NCCLS:
-
National Committee for Clinical Laboratory Standards
- PPA:
-
Post partal age
- ROC:
-
Receiver operating characteristics
- SD:
-
Standard deviation
References
Barben J, Ammann RA, Metlagel A, Schöni MH (2005) Conductivity determined by a new sweat analyzer compared with chloride concentrations for the diagnosis of cystic fibrosis. J Pediatr 146:183–188
Barben J, Desax MC, Ammann RA, Schöni MH (2005) Limitations of sweat conductivity determinations with Nanoduct analyzing system for rapid sweat testing in patients with cystic fibrosis. Eur Respir J 26:403s, (abstract)
Beauchamp M, Lands LC (2005) Sweat-testing: a review of current technical requirements. Pediatr Pulmonol 39:507–511
Carter EP, Barrett AD, Heeley AF, Kuzemko JA (1984) Improved sweat test method for the diagnosis of cystic fibrosis. Arch Dis Child 59:919–922
Cystic Fibrosis Foundation CDC (1993) Update 1. In. Bethesda: Cystic Fibrosis Foundation
Desax MC, Barben J, Hammer J, Ammann RA, Schöni MH (2006) Nanoduct sweat conductivity measurements in 1000 subjects. J Cystic Fibrosis 5:S105, (abstract)
Desax MC, Barben J, Hammer J, Ammann RA, Schöni MH (2006) Nanoduct sweat conductivity measurements: a good method for sweat testing in patients with cystic fibrosis. Swiss Med Wkly 136(Suppl 151):38S, (abstract)
di Sant’Agnese PA, Darling RC, Perera GA, Shea E (1953) Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas: clinical significance and relationship to the disease. Pediatrics 12:549–563
Eng W, LeGrys VA, Schechter MS, Laughon MM, Barker PM (2005) Sweat-testing in preterm and full-term infants less than 6 weeks of age. Pediatr Pulmonol 40:64–67
Funk MJ, LeGrys VA (2005) Testing diagnostic tests: why size matters. J Pediatr 146:159–162
Gibson LE, Cooke RE (1959) A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23:545–549
Green A, Elborn S, Fahie-Wilson MN, Kirk JM, Wallis CE, Weller P (2003) Guidelines for the performance of the sweat test for the investigation of cystic fibrosis in the UK. In. http://www.acb.org.uk
Hammond KB, Nelson L, Gibson LE (1994) Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J Pediatr 124:255–260
Heeley ME, Woolf DA, Heeley AF (2000) Indirect measurements of sweat electrolyte concentration in the laboratory diagnosis of cystic fibrosis. Arch Dis Child 82:420–424
Knowles MR, Durie PR (2002) What is cystic fibrosis? N Engl J Med 347:439–442
LeGrys VA (1992) Assessing quality assurance for sweat chloride testing. Clin Lab Sci 5:354–357
LeGrys VA, Rosenstein BJ, Doumas BT et al (2000) Sweat testing: sample collection and quantitative analysis; approved Guideline, 2nd edn. C34-A2. National Committee for Clinical Laboratory Standards
Losty HC, Wheatley H, Doull I (2006) The evaluation of a novel conductmetric device for the diagnosis of cystic fibrosis. Ann Clin Biochem 43:375–381
Mastella G, Di Cesare G, Borruso A, Menin L, Zanolla L (2000) Reliability of sweat-testing by the Macroduct collection method combined with conductivity analysis in comparison with the classic Gibson and Cooke technique. Acta Paediatr 89:933–937
Rosenstein BJ, Cutting GR, for the Cystic Fibrosis Foundation Consensus Panel (1998) The diagnosis of cystic fibrosis: a consensus statement. J Pediatrics 132:589–595
Webster HL, Quirante CG (2000) Micro-flowcell conductometric sweat analysis for cystic fibrosis diagnosis. Ann Clin Biochem 37:399–407
Acknowledgements
We thank PD Dr. M. Nelle, Neonatology Unit Dept. of Pediatrics, University of Berne and Dr. M. Travaglini, Neonatology Unit of Lindenhofspital, Berne, for their support to perform sweat tests in newborns.
If needed the genotyping was provided by the Molecular Genetic Laboratory of the University of Berne (Prof. Sabina Gallati), University of Basel (Prof. Peter Miny) and the University of Zurich (Prof. Wolfgang Berger).
No financial support was received by the Wescor Company.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Desax, MC., Ammann, R.A., Hammer, J. et al. Nanoduct® sweat testing for rapid diagnosis in newborns, infants and children with cystic fibrosis. Eur J Pediatr 167, 299–304 (2008). https://doi.org/10.1007/s00431-007-0485-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-007-0485-0