Abstract
Introduction
Kaposi’s sarcoma (KS) is rare in childhood. It may be favored by acquired immune deficiencies, but the predisposing factors to KS in other children are unclear.
Discussion
KS has been reported in only two children and one adult with primary immunodeficiency. We report here a Tunisian child with a Wiskott-Aldrich syndrome who developed KS at the age of 14 months.
Conclusion
This observation expands the spectrum of primary immunodeficiencies associated with KS in childhood.
Similar content being viewed by others
Introduction
Kaposi’s sarcoma (KS) was first described by Moritz Kaposi in 1872 [14]. It is an angiogenic-inflammatory neoplasm that originates from lymphatic endothelial cells and is driven by human herpesvirus-8 (HHV-8, also known as Kaposi’s sarcoma-associated herpesvirus) [2, 20]. Four clinical and epidemiological forms of KS have been defined: two idiopathic forms—the Sub-Saharan African (endemic) and Mediterranean (classic) forms [4]—and two secondary forms associated with either human immunodeficiency virus (HIV) co-infection (epidemic) [4] or transplantation-related immunosuppression (iatrogenic) [18]. KS is rare in children, but the number of reported cases of secondary forms has recently increased due to the HIV pandemic [28] and the increasingly frequent use of immunosuppressive drugs [27]. Only 29 cases of the classic form of KS have been reported to date in children living around, or originating from, the Mediterranean Basin [1, 3, 9–11, 17, 29]. A few cases of KS have been associated with primary immunodeficiencies (PIDs) [21]. These few cases of KS are surprising because PIDs affect a variety of immune cells and molecular pathways and at least some of these conditions affect T cells. We report here the first case of well-documented classic KS in a child with Wiskott-Aldrich syndrome (WAS).
Case report
The boy was born in 2000 to nonconsanguineous Tunisian parents living in Tunisia. His parents and sister were healthy. He was diagnosed at 23 months with Wiskott-Aldrich syndrome, due to a small WAS deletion (422del6) (nucleotide numbering according to http://www.homepage.mac.com/kohsukeimai/wasp/WASPbase.html). This mutation in exon 4 is in frame and is responsible for the 130Asp-Glu131 deletion in the WH1 domain of the WASP protein. Moreover, there was no detectable WASP protein in the patient’s PBMC using the protein assays described by Qasim et al. [24]. The patient’s medical history was marked by a local infection caused by bacille Calmette-Guérin vaccine and by chronic eczema from the age of 2 months, requiring 2 months of local steroid treatment. At 13 months, he suffered from thrombocytopenia (25×109 platelets/l), with reduced mean platelet volume, complicated by upper intestinal bleeding, which responded to blood transfusions and systemic corticosteroid treatment for 1 month (1 mg kg−1 day−1). He presented with severe staphylococcal pneumonia requiring mechanical ventilation at the age of 14 months. At 19 months, he suffered from autoimmune hemolytic anemia, which was treated by systemic corticosteroid treatment at 2 mg kg−1 day−1 for 2 months, then 3 mg kg−1 day−1 for another month.
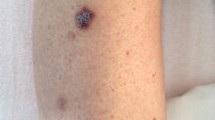
At the age of 14 months, he developed multiple, small, painless, apparently angiomatous lesions on the anterior surfaces of the groin, chest and head. Six months later, the lesions had continued to progress, increasing in size and number. The oldest lesions were impressive, nodular and firm, and were dark blue. Some tumors appeared hyperkeratotic. Edema on the surrounding tissue could be observed, and on the dorsum of the fingers it antedated the cutaneous lesions. Non-scaly, reddish brown, well-circumscribed violaceous patches appeared on the patient’s face, legs and hands (Fig. 1a,b). The oral cavity was not involved. Clinical examination revealed hepatomegaly, splenomegaly, and enlarged bilateral cervical, submandibular, axillar and inguinal lymph nodes. Cerebral CT scan revealed a left intra-orbital lesion. A CT scan of the chest showed bilateral mediastinal and hilar adenopathies. Abdominal ultrasound revealed an abdominal mass of lymph nodes in the right flank, with homogeneous hepatomegaly. The relevant immunologic data are summarized in the Table 1. At 23 months of age, the patient was transferred into the Pediatric Immunology Hematology unit at the Necker Hospital in Paris, France.
The histological diagnosis of KS was performed at the age of 23 months on skin lesions and lymph-node biopsies. Pathologic analysis of a dermal and subcutaneous ulcerative skin showed numerous irregular blood vessels and the appearance of spindle-shaped cells in the background. The spindle cells formed slit-like spaces occupied by red blood cells. Immunohistochemistry showed that spindle cells were positive for CD34 and negative for PS-100 (Fig. 2a). These cells were also positive for HHV-8-associated latent nuclear antigen LANA (ORF73), confirming the diagnosis of KS (Fig. 2b). Antibodies against both latent and lytic antigens were sought by means of two specific immunofluorescence tests, as previously described [23]. The patient had high titers of antibodies against lytic antigens (1/320) but not against latent antigens (<1/20), whereas both types of antibody were detected at a low level (1/40 and 1/20, respectively) in the patient’s mother. Quantification of HHV-8 was performed by real-time polymerase chain reaction (TaqMan), as previously described [22]. HHV-8 viral load was 0.007 copies per cell for buffy-coat DNA, 0.0017 copies per cell in bone-marrow cells, 0.302 copies per cell in the lymph node and 0.117 copies per cell in the skin biopsy sample. The 870-bp ORF K1-coding region from nucleotide positions 105–974, numbered as for strain BC1 [25], was amplified, both in the lymph node and the skin biopsy DNA, directly by polymerase chain reaction with Ampli-Taq Gold (Perkin Elmer) as a 1,073-bp fragment, as previously described [16]. Phylogenetic analyses provided evidence for infection by an HHV-8 strain of the ORF K1 C molecular subtype C′′ subgroup. Serologic assays for Epstein-Barr virus (EBV), cytomegalovirus (CMV) and herpes simplex virus were positive, whereas tests for HHV-6, HIV-1, HIV-2, HTLV-1 and HTLV-2 were negative.
At the age of 24 months, taxol treatment was immediately initiated at a dose of 60 mg/m2 given every 3 weeks in three infusions. The cutaneous lesions stabilized within 2 weeks, and partial regression was observed 1 month after the initiation of treatment (to be reported elsewhere). At the same time the patient presented with intracerebral EBV-related lymphoproliferative disease, which was treated with anti-CD20 antibody and by chemotherapy. Coinfection with CMV was treated simultaneously with ganciclovir. At the age of 28 months, the patient underwent non-T-cell-depleted allogeneic hematopoietic stem-cell transplantation (HSCT) after conventional conditioning. The donor was the patient’s father who is HLA-non-identical with HLA class I A mismatch. Stable engraftment with only donor chimerism on CD3+ cells was observed. Splenectomy was performed 5 months after HSCT for persistent peripheral thrombocytopenia. One year after HSCT, all KS cutaneous lesions, lymph nodes and hepatomegaly had disappeared, and the patient was in full remission. At last follow-up, 45 months after HSCT, the patient was alive and well, off treatment, with stable chimerism.
Discussion
The patient with WAS reported here presented HHV-8-associated KS. KS was diagnosed in this case on the basis of the following five criteria: typical clinical course, typical histopathological lesions, detection of HHV-8 antigen in situ, presence of HHV-8-specific serum antibodies, and detection of HHV-8 DNA in a cutaneous lesion. The Tunisian origin of the child and the C subtype of the HHV-8 strain identified are also consistent with a diagnosis of Mediterranean classic KS. The disease was disseminated to the skin, lymph nodes, liver and spleen. Taxol treatment gave partial remission of the KS and subsequent HSCT cured WAS and has contributed to the control of KS, as well as EBV-lymphoproliferative disorder in this patient.
WAS is a rare X-linked immunodeficiency disorder characterized by thrombocytopenia and small platelets, eczema, recurrent infections, and increased risk for autoimmunity and malignancy [8, 13]. Patients with WAS are highly susceptible to diseases caused by herpesviruses [26], and to EBV-lymphoproliferative disorders in particular [13]. One young adult with WAS and KS has been reported [19]. The HHV-8 status of this patient was not established, and he also had concomitant T-cell lymphoma, which may have contributed to the development of KS. Our patient had received systemic steroid treatment for 1 month and a first blood transfusion. Only 1 month after, KS lesions appeared at the age of 14 months. Systemic steroid treatment may have partially contributed to the development of KS. However, KS appeared before the onset of EBV-lymphoproliferative disease and the use of other systemic immunosuppressive drugs. Our patient is the second patient to be identified with both WAS and KS, but the first child. This observation expands the spectrum of PID associated with KS and supports the existence of a relationship between WAS and KS. A diagnosis of KS should be considered in selected children with WAS presenting with evocative cutaneous lesions.
KS associated with PID has been previously reported in only two children with PIDs, one child with complete IFNγR1 deficiency and mycobacterial coinfection [5] and in one child with an ill-defined PID associated with profound CD4 lymphopenia [15]. Why is KS so uncommon in children with PIDs, and why are PIDs so rare among children with KS? Classic KS is rare in childhood, with only 29 reported cases to date [1, 3, 9–11, 17, 29]. Only two multiplex families with KS have been reported, in a mother and her child [30] and in four adult siblings [12]. Together with the two multiplex families, the three observations of PIDs associated with KS are consistent with the notion that “idiopathic” childhood and perhaps even adulthood KS may actually result from nonconventional primary immunodeficiencies, presenting as predispositions to KS displaying Mendelian inheritance [6, 7].
Abbreviations
- KS:
-
Kaposi’s sarcoma
- HHV-8:
-
Human herpesvirus-8
- HIV:
-
Human immunodeficiency virus
- PIDs:
-
Primary immunodeficiencies
- WAS:
-
Wiskott-Aldrich syndrome
- HSCT:
-
Hematopoietic stem-cell transplantation
- EBV:
-
Epstein-Barr virus
References
Akman ES, Ertem U, Tankal V, Pamir A, Tuncer AM, Uluoglu O (1989) Aggressive Kaposi’s sarcoma in children: a case report. Turk J Pediatr 31:297–303
Antman K, Chang Y (2000) Kaposi’s sarcoma. N Engl J Med 342:1027–1038
Bisceglia M, Amini M, Bosman C (1988) Primary Kaposi’s sarcoma of the lymph node in children. Cancer 61:1715–1718
Boshoff C, Weiss RA (2001) Epidemiology and pathogenesis of Kaposi’s sarcoma-associated herpesvirus. Phil Trans R Soc Lond B Biol Sci 356:517–534
Camcioglu Y, Picard C, Lacoste V, Dupuis S, Akcakaya N, Cokura H, Kaner G, Demirkesen C, Plancoulaine S, Emile JF, Gessain A, Casanova JL (2004) HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr 144:519–523
Casanova JL, Abel L (2005) Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med 202:197–201
Casanova JL, Fieschi C, Bustamante J, Reichenbach J, Remus N, von Bernuth H, Picard C (2005) From idiopathic infectious diseases to novel primary immunodeficiencies. J Allergy Clin Immunol 116:426–430
Conley ME, Saragoussi D, Notarangelo L, Etzioni A, Casanova JL (2003) An international study examining therapeutic options used in treatment of Wiskott-Aldrich syndrome. Clin Immunol 109:272–7
Dutz W, Stout S (1960) Kaposi’s sarcoma in infants and children. Cancer 13:684–694
Erdem T, Atasoy M, Akdeniz N, Parlak M, Ozdemir S (1999) A juvenile case of classic Kaposi’s sarcoma. Acta Derm Venereol 79:492–493
Ferrari A, Casanova M, Bisogno G, Cecchetto G, Meazza C, Gandola L, Garaventa A, Mattke A, Treuner J, Carli M (2002) Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol 39:109–1014
Guttman-Yassky E, Cohen A, Kra-Oz Z, Friedman-Birnbaum R, Sprecher E, Zaltzman N, Friedman E, Silbermann M, Rubin D, Linn S, Whitby D, Gideoni O, Pollack S, Bergman R, Sarid R (2004) Familial clustering of classic Kaposi sarcoma. J Infect Dis 189:2023–2026
Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S (2004) Clinical course of patients with WASP gene mutations. Blood 103:456–464
Kaposi M (1872) Idiopathisches multiples Pigment-sarkom der Haut. Arch Dermat U Syph 4:265–273
Kusenbach G, Rubben A, Schneider EM, Barker M, Bussing A, Lassay L, Skopnik H, Heiman G (1997) Herpes virus (KSHV) associated Kaposi sarcoma in a 3-year-old child with non-HIV-induced immunodeficiency. Eur J Pediatr 156:440–443
Lacoste V, Judde JG, Briere J, Tulliez M, Garin B, Kassa-Kelembho E, Morvan J, Couppie P, Clyti E, Forteza Vila J, Rio B, Delmer A, Mauclere P, Gessain A (2000) Molecular epidemiology of human herpesvirus 8 in africa: both B and A5 K1 genotypes, as well as the M and P genotypes of K14.1/K15 loci, are frequent and widespread. Virology 278:60–74
Landau HJ, Poiesz BJ, Dube S, Bogart JA, Weiner LB, Souid AK (2001) Classic Kaposi’s sarcoma associated with human herpesvirus 8 infection in a 13-year-old male: a case report. Clin Cancer Res 7:2263–2268
Mendez JC, Procop GW, Espy MJ, Smith TF, McGregor CG, Paya CV (1999) Relationship of HHV8 replication and Kaposi’s sarcoma after solid organ transplantation. Transplantation 67:1200–1201
Meropol NJ, Hicks D, Brooks JJ, Siminovitch KA, Fishman NO, Kant JA, Bennett JS (1992) Coincident Kaposi sarcoma and T-cell lymphoma in a patient with the Wiskott-Aldrich syndrome. Am J Hematol 40:126–134
Moore PS, Chang Y (1995) Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med 332:1181–1185
Notarangelo L, Casanova JL, Fischer A, Puck J, Rosen F, Seger R, Geha R (2004) Primary immunodeficiency diseases: an update. J Allergy Clin Immunol 114:677–687
Oksenhendler E, Carcelain G, Aoki Y, Boulanger E, Maillard A, Clauvel JP, Agbalika F (2000) High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 96:2069–2073
Plancoulaine S, Abel L, van Beveren M, Gessain A (2002) High titers of anti-human herpesvirus 8 antibodies in elderly males in an endemic population. J Natl Cancer Inst 94:1333–1335
Qasim W, Gilmour KG, Heath S, Ashton E, Cranston T, Thomas A, Finn A, Davies EG, Thrasher AJ, Kinnon C, Jones A, Gaspar HB (2001) Potein assays for diagnosis of Wiskott-Aldrich syndrome and X-linked thrombocytopenia. Br J Haematol 113:861–865
Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS (1996) Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc Natl Acad Sci USA 93:14862–14867
Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA (1994) A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr 125:876–885
Tamariz-Martel R, Maldonado MS, Carrillo R, Crespo D, Perez-Caballero C, Munoz A (2000) Kaposi’s sarcoma after allogeneic bone marrow transplantation in a child. Haematologica 85:884–885
Ziegler JL, Katongole-Mbidde E (1996) Kaposi’s sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer 65:200–203
Zurrida S, Agresti R, Cefalo G (1994) Juvenile classic Kaposi’s sarcoma: a report of two cases, one with family history. Pediatr Hematol Oncol 11:409–416
Zurrida S, Bartoli C, Nole F, Agresti R, Del Prato I, Colleoni M, Bajetta E (1992) Classic Kaposi’s sarcoma: a review of 90 cases. J Dermatol 19:548–552
Acknowledgements
We would like to thank Nicole Brousse and Laurent Abel for helpful discussions. We would like to thank all members of the Pediatric Immunology Hematology unit who took care of the patient. We would like to thank Alexis Proust for skillful technical assistance. R. Duprez was supported by the Ligue contre le Cancer. This work was supported by the Fondation BNP-Paribas and Fondation Schlumberger.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Picard, C., Mellouli, F., Duprez, R. et al. Kaposi’s sarcoma in a child with Wiskott-Aldrich syndrome. Eur J Pediatr 165, 453–457 (2006). https://doi.org/10.1007/s00431-006-0107-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0107-2