Abstract
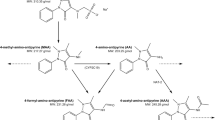
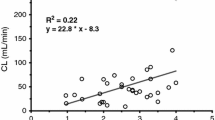
Piritramide is indicated for treatment of postoperative pain and analgosedation in the intensive care unit (ICU) setting. In an open prospective study the pharmacokinetics of piritramide were investigated in four groups: newborns (NB, age: 1–28 days) (n=8), infants 1 (IF1, age: 2–4 months) (n=7), infants 2 (IF2, age: 5–12 months) (n=14) and young children (YC, age: 2–4 years) (n=10). The recommended paediatric dose range for therapy of postoperative pain is 50–200 μg/kg. Piritramide was administered intravenously as a single dose by bolus injection of 50 μg/kg. Blood samples were collected at 0, 15, 45, 90 min and 3, 6, 9, 12 h after application, and urine samples were collected before application and during the following intervals: 1–2, 2–6, 6–12 h. Piritramide was measured in blood and urine by HPLC-ESI-MS. The following pharmacokinetic parameters: maximum plasma concentration (Cmax), distribution half-life \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} } \right)},\), elimination half-life\({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} } \right)},\), total clearance (Clt) and median volume of distribution at equilibrium (Vdss) were calculated using a non-compartment and a two-compartment model for the disposition of piritramide (TOPFIT and NONMEM-pharmacokinetic analysis). Newborns (NB) showed the highest maximum plasma concentrations (median±SD) Cmax (79±240 μg/l) compared to the other three groups (IF1 36±367, IF2 12±81 and YC 16±9 μg/l) without statistical significance. The median elimination half-lives \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {{\text{2 $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {{\text{2 $ \beta $ }}}}} } \right)}\) were 702±720 min in NB, 157±102 min in IF1, 160±68 min in IF2 and 166±143 min in YC. For \(t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {{\text{2 $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {{\text{2 $ \beta $ }}}}} \) the difference between NB and the other three groups (IF1, IF2 and YC) was statistically significant (Mann-Whitney-U, P<0.05). Clt was 15.9±16.7, 46.6±76.9, 235.5±454.1 and 338±168.1 ml/min in NB, IF1, IF2 and YC respectively. The total clearance increased exponentially with an elimination half-life of 702 min from 15.9 ml/min in NB to 46.6 ml/min in IF2. Differences between the NB/IF1 groups and IF2/YC groups were significantly significant (NB vs. IF2, NB vs. YC, IF1 vs. IF2 and IF1 vs. YC). Vdss was 2.0±4.93, 1.7±2.5, 7.0±5.2 and 6.7±2.2 l/kg in NB, IF1, IF2 and YC respectively. In comparison to group IF1 the Vdss was significantly larger in groups IF2 and YC (Mann-Whitney U, P<0.05). Newborns showed a high initial concentration and a distinct prolongation of the elimination half-life of piritramide compared to infants, young children and adults. Therefore, dosage needed to treat postoperative pain should be reduced, and the repetitive doses should be geared to the analgesic effects. In infants and young children the elimination of piritramide is increased compared to adults; therefore the duration of the effects of piritramide will be shortened, and dose intervals ought to be reduced. Subsequent clinical trials for detailed dose adjustment of piritramide in paediatric patients comparing pharmacokinetics and effectiveness are needed.








Similar content being viewed by others
Abbreviations
- ICU:
-
Intensive care unit
- Cmax :
-
Highest observed serum concentration (μg/l)
- i.v.:
-
Intravenous
- HPLC:
-
High performance liquid chromatography
- \(t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} \) :
-
Half-life of drug during distribution (min)
- \(t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} \) :
-
Half-life of drug during terminal phase (min)
- Vdss :
-
Steady-state volume of distribution after intravenous administration (l)
- PIR:
-
Piritramide
- PK:
-
Pharmacokinetics
- SD:
-
Standard deviation
References
Anon (1991) Piritramide (monograph 778). Drugs available abroad. A guide to therapeutic drugs available and approved outside the U.S. Derwent Publications, London, UK
Beal SL, Sheiner LB, Boekmann A (1998) NONMEM user’s guide. Division of Pharmacology, University of California, San Francisco
Blumer JL (ed) (1999) The therapeutic orphan-30 years later. Proceedings of a joint conference of the Pediatric Pharmacology Research Unit Network, the European Society of Developmental Pharmacology, and the National Institute of Child Health and Human Development. Pediatrics 104(3 Pt 2):581–645
Bouillon T, Groeger P, Kietzmann D (2004) The pharmacokinetics of piritramide after prolonged administration to intensive care patients. Eur J Anaesth 21:673–678
Boullion T, Kietzmann D, Port R, Meineke I, Hoeft A (1999) Population pharmacokinetics of piritramide in surgical patients. Anesthesiology 90:7–15
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NHG (2004) Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92(2):208–217
Brack A, Böttiger BW, Schäfer M (2004) New insights of postoperative pain therapy. Anästhesiol Intensivmed Notfallmed Schmerzther 39:157–164
Büttner W (1994) Die Erfassung des postoperativen Schmerzes beim Kleinkind. Habilitations-Schrift, Ruhr-Universität Bochum. Arcis-Verlag, Munich
Büttner W, Finke W (2000) Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth 10:303–318
Büttner W, Finke W, Schwanitz M (1990) Nalbuphine and piritramide in the postoperative period in small children. Part 2. Breathing patterns. Anaesthesist 39:258–263
Dagan O, Klein J, Bohn D, Barker G, Koren G (1993) Morphine pharmacokinetics in children following cardiac surgery: effects of disease and inotropic support. J Cardiothorac Vasc Anesth 7(4):396–398
Davis PJ, Killian A, Stiller RL, Cook DR, Guthrie RD, Scierka AM (1989) Pharmacokinetics of alfentanil in newborn premature infants and older children. Dev Pharmacol Ther 13(1):21–27
Hutchinson TA. Shahan DR (2004) Drugdex system. Healthcare series vol. 121. Micromedex, Greenwood Village, CO
Fachinformation (2002) Dipidolor. Bundesverband der Pharmazeutischen Industrie
Gauntlett IS, Fisher DM, Hertzka RE, Kuhls E, Spellman MJ, Rudolph C (1988) Pharmacokinetics of fentanyl in neonatal humans and lambs: effects of age. Anesthesiology 69:683–687
Gladtke E (1979) The importance of pharmacokinetics for paediatrics. Eur J Pediatr 131(2):85–91
Gow PJ, Ghabrial H, Smallwood RA, Morgan DJ, Ching MS (2001) Neonatal hepatic drug elimination. Pharmacol Toxicol 88(1):3–15
Greeley WJ, de Bruin NP, Davis DP (1987) Sufentanil pharmacokinetics in pediatric cardiovascular patients. Anesth Analg 66:1067–1072
Guay J, Gaudreault P, Tang A, Goulet B, Varin F (1992) Pharmacokinetics of sufentanil in normal children. Can J Anaesth 39:14–20
Hartwig S, Roth B, Theisohn M (1991) Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. Eur J Pediatr 150(11):784–788
Heinzel G, Wolosczak R, Thomann P (1993) TopFit version 2.0: pharmacokinetic and pharmacodynamic data analysis system. Gustav Fischer Verlag, Stuttgart
Henschel WF, Buhr G, Fernanadez R (1968) Clinical tests with a new long acting analgesic. In: Progress in anaesthesiology (Proceedings of the fourth World Congress of Anaesthesiologists), Amsterdam, Excerpta Medica International Congress Series No 200 74:887
Höhne C, Donaubauer B, Kaisers U (2004) Opioids during anesthesia in liver and renal failure. Anaesthesist 53:291–303
Hughes M, Glass P, Jacobs J (1992) Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology 76:334–341
Hunt A, Joel S, Dick G, Goldman A (1999) Population pharmacokinetics of oral morphine and its glucuronids in children receiving morphine as immediate-release liquid or sustained-release tablets for cancer pain. J Pediatr 135:47-55
Jacqz-Aigrain E, Burtin P (1996) Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet 31:423–443
Janssen PAJ (1982) Potent, new analgesics, tailor-made for different purposes. Acta Anaesth Scand 26:262–268
Janssen PAJ (1961) Piritramide (R 3365) a potent analgesic with unusual chemical structure. J Pharm Pharmacol 13:513–530
Kart T, Christrup LL, Rasmussen M (1997) Recommended use of morphine in neonates, infants and children based on a literature review. Part 1—pharmacokinetics. Paediatr Anaesth 7(1):5–11
Kay B (1971) A clinical investigation of piritramide in the treatment of postoperative pain. Br J Anaesth 43:1167–1171
Kietzmann D, Bouillon T, Hamm C, Schwabe K, Schenk H, Gundert-Remy U, Kettler D (1997) Pharmacodynamic modelling of the analgesic effects of piritramide in postoperative patients. Acta Anaesthesiol Scand 41:888–894
Kietzmann D, Briede I, Bouillon T, Gundert-Remy U, Kettler D (1996) Pharmacokinetics of piritramide after an intravenous bolus in surgical patients. Acta Anaesthesiol Scand 40:898–903
Kuhls E, Gauntlett IS, Lau M, Brown R, Rudolph CD, Teitel DF, Fisher DM (1995) Effect of increased intra-abdominal pressure on hepatic extraction and clearance of fentanyl in neonatal lambs. J Pharmacol Exp Ther 274(1):115–119
Kumar N, Rowbotham DJ (1999) Editorial II. Piritramide. Br J Anaesth 82(1):3–5
Latasch L, Freye E (2002) Pain and opioids in the preterm and the neonate. Anaesthesist 51:272–284
Martens-Lobenhoffer L, Römhild W (2003) Quantitative determination of piritramide in human serum applying liquid chromatography-two-stage mass spectrometry. J Chromatogr B 783:53–59
Michaelis HC, Kietzmann D, Neurath H, Jongepieper U, Schilling B (1991) Sensitive determination of piritramide in human plasma by gas chromatography. J Chromatogr 571:257–262
Misztal G (1991) Determination of piritramide in plasma using high pressure liquid chromatography methods. Acta Pol Pharm 48(5–6):1–2
Saarenmaa E, Neuvonen PJ, Fellman V (2000) Gestational age and birth effects on plasma clearance of fentanyl in newborn infants. J Pediatr 136(6):767–770
Saarne A (1969) Clinical evaluation of the new analgesic piritramide. Acta Anaesth Scand 13:11–19
Taddio A (2002) Opioid analgesia for infants in the neonatal intensive care unit. Clin Perinatol 29:493–509
Weyne F, Schluter J, Lust P (1968) Piritramide, a potent postoperative analgesic with unusually low respiratory depressant, cardiovascular and emetic effects. Acta Anaesth Belg 19:33–45
Acknowledgement
The authors appreciated strongly the help of the nurses and physicians of the Children’s hospitals to maintain the study, filling out the case report formulas and giving support and general assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, C., Kremer, W., Harlfinger, S. et al. Pharmacokinetics of piritramide in newborns, infants and young children in intensive care units. Eur J Pediatr 165, 229–239 (2006). https://doi.org/10.1007/s00431-005-0021-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-005-0021-z