Abstract
The circulation of H9N2 viruses throughout the world, along with their expanded host range, poses a potential health risk to the public, but the host responses to H9N2 virus in mammals were little known. To obtain insight into the host immune responses to the avian H9N2 virus, the expressions of both cytokines and chemokines in the lungs of infected mice were examined by real-time polymerase chain reaction and enzyme-linked immunosorbent assay. We found that interferon gamma (IFN-γ) was the dominant antiviral component, and IFN-γ-induced protein 10 kDa, interleukin 6, chemokine (C–C motif) ligand 5 and macrophage inflammatory protein-1 alpha all played a role in pro-inflammatory responses to H9N2 viruses. In conclusion, this research can make us further understand the infection characteristics of H9N2 virus in mammalian host by providing the data on mice lung immune responses to the avian H9N2 virus.
Similar content being viewed by others
Introduction
H9N2 avian influenza virus (AIV) has been circulating worldwide in many avian species and resulted in great economic losses [1–3]. More importantly, human cases of avian H9N2 virus infection have been reported in Hong Kong and mainland China since the late 1990s [4–6], and seroprevalence investigations of H9N2 in poultry workers were also the solid evidence on human cases of H9N2 infection [7–9]. The above-mentioned researches have intrigued a great concern of the public.
H9N2 infection in mammals mainly depends on the ability of the virus to bind the human-like α2,6-linked sialic acid (SA-α2,6) receptors, and the HA receptor-binding site is critical for virus host range [10–12]. Some isolates of the H9N2 influenza viruses circulating in poultry can infect humans due to the ability to binding the SA-α 2,6 receptors [10, 13]. The expanded receptor specificity of H9N2 AIVs has raised concerns about their pathogenicity in humans.
H9N2 influenza viruses of chicken origin cause only mild symptoms in humans, but they have the pandemic potential for the high level of genetic plasticity [14–16]. For example, sequencing analyses of the novel influenza A (H7N9) virus isolated in China showed that 6 out of 8 fragments were from H9N2 [17]. Facing the threat of H9N2 AIV, understanding the mammalian host immune responses to the virus is of importance to cope with the possible pandemic. However, up to date, little information about mammal immune responses to the H9N2 virus of chicken origin was reported.
To fill the literature gap, therefore, the expression of six cytokines [interferon beta (IFN) β, interferon gamma (IFN-γ), tumor necrosis factor (TNF) α, interleukin (IL) 1β, IL-6, IL-10] and five chemokines [IFN-γ-induced protein 10 kDa (IP-10), chemokine (CC motif) ligand 5 (CCL-5), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1α), IL-8] were evaluated in the lungs of H9N2-infected mice with the aim to make us further understand the infection characteristics of H9N2 AIV in mammalian host.
Materials and methods
Ethics statement
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Shandong Agricultural University and performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China). Animal suffering was minimized as much as possible.
Virus
The H9N2 virus, A/chicken/Shandong/w3/11/H9N2 (SDw3), belonging to the BJ94-like lineage was isolated from diseased chicken in Shandong. The virus was passaged in 10-day-old specific pathogen-free (SPF) chicken embryos. And the 50 % tissue-culture-infective dose (TCID50) were calculated by the method of Reed and Muench [18].
Mice experiments
Twenty-eight SPF female BALB/c mice (18.0–20.0 g, 6–8 weeks) from Experimental Animal Center of Shandong Province were randomly divided into the infected group and the control group. After being lightly anesthetized with CO2, the mice were inoculated intranasally with 106 TCID50 of SDw3 in fifty microliters of phosphate-buffered saline (PBS) or PBS alone.
At 0.5, 2 and 6 days post-inoculation (dpi), lungs, hearts, livers, spleens, kidneys and brains of mice (3/group) in the infected group and the control were, respectively, collected for detecting SDw3 virus titers. Simultaneously, the mice lungs were used for cytokines and chemokines detection by real-time polymerase chain reaction (PCR) or enzyme-linked immunosorbent assay (ELISA) [19]. Additionally, the mouse lungs collected at 5 dpi were fixed in 10 % phosphate-buffered formalin, embedded in paraffin, then cut into 5-mm-thick sections and stained with hematoxylin and eosin (H&E) [20]. During the raising period, the weight loss and clinical signs of the mice were monitored and recorded. After the experiment, the mice were killed due to ethical reasons.
RNA extraction, cDNA preparation and real-time PCR
The RNA of the lung samples was extracted using an RNeasy Mini Kit (Qiagen, Valencia, USA). The quantity and quality of the isolated RNA were determined by UV 260/280 using a biophotometer (Eppendorf, Hamburg, Germany). A total of 500 ng RNA was used to prepare cDNA by the reverse transcription reaction with a PrimeScript RT reagent Kit (TaKaRa, Dalian, China).
Real-time PCR was performed using SYBR Premix Ex Taq kit (TaKaRa, Dalian, China) and a standard 7500 Real-Time PCR System (Applied Biosystems, Foster City, USA) to obtain the relative expression quantity of message RNAs (mRNAs) of six cytokines and five chemokines (β-action was used as housekeeping genes) [21, 22].
Except that the primers (IFN-β, TNF-α, IL-1β, IL-10, MCP-1, MIP-1α and β-action) were cited according to the published references [23, 24], the other primers (Table 1) designed using Premier 5.0 software (Applied Biosystems, Foster City, USA).
Enzyme-linked immunosorbent assay
The protein levels of cytokines and chemokines in lung homogenates were measured using the mouse Quantikine Kits (RayBiotech Inc., Norcross, GA, USA; R&D Systems, Minneapolis, MN, USA). Briefly, the entire lung of each mouse was homogenized individually in 500 μl Cell/tissue lysis buffer (RayBiotech Inc., Norcross, GA). After centrifugation at 10,000g for 15 min, the supernatants were collected for analyzing cytokines and chemokines simultaneously according to the kit instructions. The plates were read on a spectrophotometer at wavelength 450 nm by a microplate reader (Bio-Rad, Richmond, CA, USA). Finally, the cytokine or chemokine levels were recorded as ng/ml homogenate.
Statistical analysis
Statistical analysis was performed using the Statistical Product and Services Solutions, version 10.0 (SPSS, Cary, NC, USA). ANOVA analysis was performed by comparing infected data to uninfected data. A p value <0.05 was considered statistically significant.
Results
Pathogenicity of SDw3 in mice
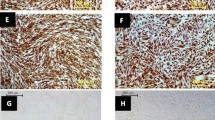
Upon being injected with SDw3, the mice showed mild signs of illness, but they recovered naturally at 6 dpi. The body weight of infected mice dropped lightly during the first 3 days, but they almost regained the original body weight at 6 dpi (Fig. 1a). The virus titer was detectable only in the mice lungs, with a peak at 2 dpi (Fig. 1b). H&E staining of the infected mice lungs (5 dpi) showed that SDw3 caused mild and limited interstitial pneumonia; the interstitial tissue was lightly thickened and filled with immune cells and inflammatory compared with the mock lungs (Fig. 1c).
Virulence of SDw3 in mice. Mice were inoculated intranasally with 106 TCID50 of SDw3 or PBS. a Body weight were monitored and recorded daily. b Virus titers in lungs of SDw3-infected mice. The lungs of SDw3-infected mice (n = 3 per time point) were collected at 0.5, 2 and 6 dpi for virus titration. Virus titers were given in units of log10 TCID50 per g. Bars represent mean ± standard deviation (SD) of three mice. c Histopathology of mock (left) and SDw3-infected (right) mice. Lungs were collected at 5 dpi and fixed in 10 % formalin, embedded in paraffin and sectioned (magnification ×100)
Cytokine/chemokine mRNA expression
After infection, there was a two- to 20-fold mRNA induction of IFN-γ, IL-6, IP-10, CCL-5 and MIP-1α; the concentrations of CCL-5 and IFN-γ were, respectively, increased up to over 15-fold at 2 dpi and 20-fold at 6 dpi (Fig. 2). The data showed the peak induction for most of the mRNAs (IL-6, IP-10, CCL-5 and MIP-1α) occurred at 2 dpi, except for IFN-γ which was most increased at 6 dpi. By contrast, the other mRNAs were not up-regulated significantly (data not shown).
Cytokine and chemokine mRNA response of mouse lung to H9N2. Cytokine and chemokine mRNA expressions in lungs of mice (n = 3 per time point) were measured by real-time PCR and were presented as the mean fold change (±SD) compared with values in mock mice at the days after inoculation. Statistical analysis was performed by comparing with the data of mock mice. Asterisk p < 0.05 between mock and SDw3-infected mice
Cytokine/chemokine protein profiles
Protein expressions of the five corresponding cytokines and chemokines (IFN-γ, IL-6, IP-10, CCL-5, and MIP-1α) were confirmed by ELISA, and their protein levels were all above the control at 0.5 dpi (Fig. 3). Protein expressions of IFN-γ, CCL-5 and MIP-1α peaked at 2 dpi, whereas IL-6 at 6 dpi.
H9N2 stimulates cytokine and chemokine release in mouse lung. Mice were inoculated intranasally with 106 TCID50 of SDw3 or PBS. The lungs of SDw3-infected mice (n = 3 per time point) were harvested and homogenized in 500 μl of Lysis Buffer. The protein of cytokines and chemokines were measured by ELISA. Baseline protein levels of cytokines and chemokines from PBS-inoculated mice (n = 5) were shown as a dashed line in each graph. The data was presented as mean ±SD for three independent experiments. Asterisk p < 0.05 between mock and SDw3-infected mice
Discussion
SDw3, the dominant viral isolate in China, belongs to the BJ94-like lineage [25, 26]. The pathogenicity result of this study showed that the virus was replicable and pathogenic in mice without prior adaptation, which is consistent with the previous experiments [27, 28]. This was the main reason for us to further understand the immune responses of mice challenged by the virus. During the viral infection, body weight and clinical signs of mice changed lightly mainly due to the mild pathogenicity of the SDw3.
Cytokines paly an important role in clearance of virus. For example, IFN-γ mediates the production of nitric oxide, subsequently resulting in the recruitment of more neutrophils and macrophages [29, 30]; IL-6 is a multifunctional cytokine that not only regulates immune and inflammatory responses involved in the activation, growth and differentiation of T-cells, but also contributes to T cell-mediated inflammatory reactions [31]. The analyses of cytokine in this study showed that IFN-γ and IL-6 were both up-regulated in H9N2-infected mice lungs. Compared with cytokine changes in H9N2-infected chicken lungs, TNF-α, IFN-α, -β and IFN-γ were all up-regulated [32]. The differences of the breed and the immune system may be the major contributors. During the influenza virus infection, the production of chemokines, including MCP-1, MIP-1, MIP-1α, CCL-5I, IP-10 and CCL-5, was all or partly up-regulated [33, 34]. This response is crucial in pro-inflammatory responses to influenza viruses. Similarly, MIP-1α, CCL5 and IP-10 were all up-regulated in this study, which indicated that protective responses occurred in H9N2-infected mice lungs.
Time point selection of detecting cytokines and chemokines mRNA/protein expressions and observing histopathology changes was based on previous references [19, 20]. Although this selection was not perfect, it can truly reflect main change trend of cytokines and chemokines in mice lungs challenged by H9N2 influenza virus. Additionally, during the experiment, production changes of mRNA and protein at the same time point were not consistent, which can largely be attributable to the time difference between transcription and translation.
In conclusion, after infection of H9N2, IFN-γ was the dominant antiviral component, and IP-10, IL-6, CCL-5 and MIP-1α all played an important role in pro-inflammatory responses to H9N2 viruses. It is useful for us to understand the infection characteristics of H9N2 virus in mammalian host.
References
Li C, Yu K, Tian G, Yu D, Liu L et al (2005) Evolution of H9N2 influenza viruses from domestic poultry in Mainland China. Virology 340:70–83
Ducatez MF, Webster RG, Webby RJ (2008) Animal influenza epidemiology. Vaccine 26(Suppl. 4):D67–D69
Nagarajan S, Rajukumar K, Tosh C, Ramaswamy V, Purohit K, Saxena G, Behera P, Pattnaik B, Pradhan HK, Dubey SC (2009) Isolation and pathotyping of H9N2 avian influenza viruses in Indian poultry. Vet Microbiol 133:154–163
Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL et al (1999) Human infection with influenza H9N2. Lancet 354:916–917
Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH et al (2005) Human infection with an avian H9N2 influenza a virus in Hong Kong in 2003. J Clin Microbiol 43:5760–5767
Cheng VC, Chan JF, Wen X, Wu WL, Que TL et al (2011) Infection of immunocompromised patients by avian H9N2 influenza a virus. J Infect 62(5):394–399
Jia N, de Vlas SJ, Liu YX, Zhang JS, Zhan L et al (2009) Serological reports of human infections of H7 and H9 avian influenza viruses in northern China. J Clin Virol 44:225–229
Pawar SD, Tandale BV, Raut CG, Parkhi SS, Barde TD et al (2012) Avian influenza H9N2 seroprevalence among poultry workers in Pune, India, 2010. PLoS ONE 7(5):e36374
Uyeki TM, Nguyen DC, Rowe T, Lu X, Hu-Primmer J et al (2012) Seroprevalence of antibodies to avian Influenza A (H5) and A (H9) viruses among market poultry workers, Hanoi, Vietnam, 2001. PLoS ONE 7(8):e43948
Ha Y, Stevens DJ, Skehel JJ, Wiley DC (2001) X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc Natl Acad Sci USA 98:11181–11186
Imai M, Kawaoka Y (2012) The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol 2:160–167
Butt AM, Siddique S, Idrees M, Tong Y (2010) Avian influenza A (H9N2): computational molecular analysis and phylogenetic characterization of viral surface proteins isolated between 1997 and 2009 from the human population. Virol J 7:319
Matrosovich MN, Krauss S, Webster RG (2001) H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281:156–162
Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M (2000) H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol 74:9372–9380
Wan H, Sorrell EM, Song H, Hossain MJ, Ramirez-Nieto G et al (2008) Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS ONE 3:e2923
Sun Y, Qin K, Wang J, Pu J, Tang Q, Hu Y, Bi Y, Zhao X, Yang H, Shu Y, Liu J (2011) High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proc Natl Acad Sci USA 108:4164–4169
Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W (2013) Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368(20):1888–1897
Reed LJ, Muench H (1938) A simple method of estimating fifty percent end points. Am J Epidemiol 27:493–497
Maines TR, Belser JA, Gustin KM, van Hoeven N, Zeng H et al (2012) Local innate immune responses and influenza virus transmission and virulence in ferrets. J Infect Dis 205:474–485
Zhang Z, Hu S, Li Z, Wang X, Liu M, Guo Z, Li S, Xiao Y, Bi D, Jin H (2011) Multiple amino acid substitutions involved in enhanced pathogenicity of LPAI H9N2 in mice. Infect Genet Evol 11(7):1790–1797
Liang G, Chen M, Pan XL, Zheng J, Wang H (2011) Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp Toxicol Pathol 63:607–611
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Juknat A, Pietr M, Kozela E, Rimmerman N, Levy L, Coppola G et al (2012) Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Δ9-tetrahydrocannabinol in BV-2 microglial cells. Br J Pharmacol 165:2512–2528
Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G (2007) Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun 357:319–324
Xu KM, Smith GJ, Bahl J, Duan L, Tai H et al (2007) The genesis and evolution of H9N2 influenza viruses in poultry from southern China, 2000 to 2005. J Virol 81:10389–10401
Zhang Y, Yin Y, Bi Y, Wang S, Xu S et al (2012) Molecular and antigenic characterization of H9N2 avian influenza virus isolates from chicken flocks between 1998 and 2007 in China. Vet Microbiol 156:285–293
Bi J, Deng G, Dong J, Kong F, Li X et al (2010) Phylogenetic and molecular characterization of H9N2 influenza isolates from chickens in Northern China from 2007–2009. PLoS ONE 5:e13063
Deng G, Bi J, Kong F, Li X, Xu Q et al (2010) Acute respiratory distress syndrome induced by H9N2 virus in mice. Arch Virol 155:187–195
Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM (2008) H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog 4:e1000115
Karpuzoglu E, Ahmed SA (2006) Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide 15:177–186
Van Snick J (1990) Interleukin-6: an overview. Annu Rev Immunol 8:253–278
Nang NT, Lee JS, Song BM, Kang YM, Kim HS et al (2011) Induction of inflammatory cytokines and toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet Res 42:64
Xing Z, Harper R, Anunciacion J, Yang Z, Gao W et al (2011) Host immune and apoptotic responses to avian influenza virus H9N2 in human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 44:24–33
Qu B, Li X, Gao W, Sun W, Jin Y, Cardona CJ, Xing Z (2012) Human intestinal epithelial cells are susceptible to influenza virus subtype H9N2. Virus Res 163:151–159
Acknowledgments
This study was sponsored by the NSFC project (31270172); The National Science and Technology Support Project (2012BAD39B0205); Open Fund 2011 of the State Key Laboratory for Environmental Protection, Using of Environmental Microbiology and Security Controls (No. MARC2011D061); Tai’an Development Program of Science and Technology (20093061).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, R., Liu, J., Liang, W. et al. Response profiles of cytokines and chemokines against avian H9N2 influenza virus within the mouse lung. Med Microbiol Immunol 203, 109–114 (2014). https://doi.org/10.1007/s00430-013-0317-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-013-0317-y