Abstract
Leptin and glucocorticoids (GCs) are involved in metabolic functions, thymic homeostasis and immune activity through complex interactions. We recently showed that C57BL/6 mice infected with Trypanosoma cruzi revealed a fatal disease associated with a dysregulated immune–endocrine response characterized by weight loss, deleterious synthesis of pro-inflammatory cytokines and GCs-driven thymus atrophy. Extending this study, we now explored the relationship between leptin and GCs, in terms of infection outcome, thymic and metabolic changes. T. cruzi-infected mice showed a food intake reduction, together with hypoglycemia and lipolysis-related changes. Infected animals also displayed a reduction in systemic and adipose tissue levels of leptin, paralleled by a down-regulation of their receptor (ObR) in the hypothalamus. Studies in infected mice subjected to adrenalectomy (Adx) showed a worsened course of infection accompanied by even more diminished systemic and intrathymic leptin levels, for which GCs are necessary not only to decrease inflammation but also to sustain leptin secretion. Adx also protected from thymic atrophy, independently of the reduced leptin contents. Leptin administration to infected mice aggravated inflammation, lowered parasite burden and attenuated GCs release, but did not normalize thymic atrophy or metabolic parameters. Acute T. cruzi infection in C57BL/6 mice coexists with a dysregulation of leptin/hypothalamic ObR circuitry dissociated from body weight and food intake control. Endogenous GCs production attempted to reestablish systemic leptin concentrations, but failed to improve leptin-protective activities at the thymic level, suggesting that the leptin/GCs intrathymic relationship is also altered during this infection.






Similar content being viewed by others
References
Pérez AR, Bottasso O, Savino W (2009) The impact of infectious diseases upon neuroendocrine circuits. Neuroimmunomodulation 16:96–105
Bonneaud C, Mauc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–369
Moret Y, Schmid-Hempel P (2000) Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168
Buttgereit F, Burmester GR, Brand MD (2000) Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today 21(4):192–199
del Rey A, Roggero E, Randolf A, Mahuad C, McCann S, Rettori V, Besedovsky HO (2006) IL-1 resets glucose homeostasis at central levels. Proc Natl Acad Sci USA 103:16039–16044
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Kruse M, Bornstein SR, Uhlmann K, Paeth G, Scherbaum WA et al (1998) Leptin down-regulates the steroid producing system in the adrenal. Endocr Res 24:587–590
Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B (1997) Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes 46:717–719
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM (1995) Weight reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546
Matarese G, Moschos S, Mantzoros CS (2005) Leptin in immunology. J Immunol 174:3137–3142
Löllmann B, Grüninger S, Stricker-Krongrad A, Chiesi M (1997) Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and e in different mouse tissues. Biochem Biophys Res Commun 238:648–652
Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS (2003) The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421
Ahima RS, Saper CB, Flier JS, Elmquist JK (2000) Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307
Desruisseaux MS, Nagajyothi Trujillo ME, Tanowitz HB, Scherer PE (2007) Adipocyte, adipose tissue, and infectious disease. Infect Immun 75:1066–1078
Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gómez-Reino JJ, Gualillo O (2005) Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 579:295–301
La Cava A, Matarese G (2004) The weight of leptin in immunity. Nat Rev Immunol 4:371–379
Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V (2000) Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 199:15–24
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901
Raso GM, Pacilio M, Esposito E, Coppola A, Di Carlo R, Meli R (2002) Leptin potentiates IFN-gamma-induced expression of nitric oxide synthase and cyclooxygenase-2 in murine macrophage J774A. 1. Br J Pharmacol 137:799–804
De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese GA (2007) Key role of leptin in the control of regulatory T cell proliferation. Immunity 26:241–255
Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR (1999) Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104:1051–1059
Palmer G, Aurrand-Lions M, Contassot E, Talabot-Ayer D, Ducrest-Gay D, Vesin C, Chobaz-Péclat V, Busso N, Gabay C (2006) Indirect effects of leptin receptor deficiency on lymphocyte populations and immune response in db/db mice. J Immunol 177:2899–2907
Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW (2005) Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis 64:1195–1198
Zhang HH, Kumar S, Barnett AH, Eggo MC (2000) Tumour necrosis factor-alpha exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol 2000(159):79–88
Dagogo-Jack S, Selke G, Melson AK, Newcomer JW (1997) Robust leptin secretory responses to dexamethasone in obese subjects. J Clin Endocrin Metab 82:3230–3233
Halleux CM, Servais I, Reul BA, Detry R, Brichard SM (1998) Multihormonal control of ob gene expression and leptin secretion from cultured human visceral adipose tissue: increased responsiveness to glucocorticoids. J Clin Endocrino Meta 83:902–910
Miell JP, Englaro P, Blum WF (1996) Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res 28:704–707
Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Bue-Valleskey J, Stephens TW (1996) Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem 271:5301–5304
Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thorens B, Gaillard R (1998) Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology 139(10):4264–4268
Huang Q, Timofeeva E, Richard D (2006) Regulation of corticotropin-releasing factor and its types 1 and 2 receptors by leptin in rats subjected to treadmill running-induced stress. J Endocrinol 191(1):179–188
Trotter-Mayo RN, Roberts MR (2008) Leptin acts in the periphery to protect thymocytes from glucocorticoid-mediated apoptosis in the absence of weight loss. Endocrinology 149(10):5209–5218
Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, Nakao K, Mimori T (2002) Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol 128(1):21–26
Fernandes F, Dantas S, Ianni BM, Ramires FJ, Buck P, Salemi VM, Lopes HF, Mady C (2007) Leptin levels in different forms of Chagas’ disease. Braz J Med Biol Res 40:1631–1636
Tanowitz HB, Jelicks LA, Machado FS, Esper L, Qi X, Desruisseaux MS, Chua SC, Scherer PE, Nagajyothi F (2011) Adipose tissue, diabetes and Chagas disease. Adv Parasitol 76:235–250
Nagajyothi F, Desruisseaux MS, Weiss LM, Chua S, Albanese C, Machado FS, Esper L, Lisanti MP, Teixeira MM, Scherer PE, Tanowitz HB (2009) Chagas disease, adipose tissue and the metabolic syndrome. Mem Inst Oswaldo Cruz 104(1):219–225
Tanowitz HB, Amole B, Hewlett D, Wittner M (1988) Trypanosoma cruzi infection in diabetic mice. Trans R Soc Trop Med Hyg 82:90–93
Nagajyothi F, Zhao D, Machado FS, Weiss LM, Schwartz GJ, Desruisseaux MS, Zhao Y, Factor SM, Huang H, Albanese C, Teixeira MM, Scherer PE, Chua SC Jr, Tanowitz HB (2010) Crucial role of the central leptin receptor in murine Trypanosoma cruzi (Brazil strain) infection. J Infect Dis 202(7):1104–1113
Roggero E, Perez A, Tamae-Kakazu M, Piazzon I, Nepomnaschy I, Wietzerbin J, Serra E, Revelli S, Bottasso O (2002) Differential susceptibility to acute T. cruzi infection in BALB/c and C57BL/6 mice is not associated with a distinct parasite load but cytokine abnormalities. Clin Exp Immunol 128:421–428
Pérez AR, Tamae-Kakazu M, Pascutti MF, Roggero E, Serra E, Revelli S, Bottasso O (2005) Deficient control of T. cruzi infection in C57BL/6 mice is related to a delayed specific IgG response and increased macrophage production of pro-inflammatory cytokines. Life Sci 77:1945–1959
Roggero E, Piazzon I, Nepomnaschy I, Perez A, Velikovsky A, Revelli S, Bottasso O (2004) Thymocyte depletion during acute T. cruzi infection in C57BL/6 mice is partly reverted by lipopolysaccharide pretreatment. FEMS Immunol Med Microbiol 41:123–131
Pérez AR, Roggero E, Nicora A, Palazzi J, Besedovsky HO, Del Rey A, Bottasso OA (2007) Thymus atrophy during T. cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun 21:890–900
Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel AL, Houssier M, Roussel B, Besse-Patin A, Combes M, Mir L, Monbrun L, Bézaire V, Prunet-Marcassus B, Waget A, Vila I, Caspar-Bauguil S, Louche K, Marques MA, Mairal A, Renoud ML, Galitzky J, Holm C, Mouisel E, Thalamas C, Viguerie N, Sulpice T, Burcelin R, Arner P, Langin D (2013) Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 11(2):e1001485
Manarin R, Pascutti MF, Ruffino JP, de las Heras B, Boscá L, Bottasso O, Revelli S, Serra E (2010) Benznidazole blocks NF-kB Activation but not AP-1 through inhibition of IKK. Mol Immunol 47:2485–2491
Combs TP, Nagajyothi Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, Landskroner-Eiger S, Cordero A, Factor SM, Weiss LM, Lisanti MP, Tanowitz HB, Scherer PE (2005) The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem 280(25):24085–24094
Nagajyothi F, Kuliawat R, Kusminski CM, Machado FS, Desruisseaux MS, Zhao D, Schwartz GJ, Huang H, Albanese C, Lisanti MP, Singh R, Li F, Weiss LM, Factor SM, Pessin JE, Scherer PE, Tanowitz HB (2013) Alterations in glucose homeostasis in a murine model of Chagas disease. Am J Pathol 182(3):886–894
Hölscher C, Mohrs M, Dai WJ, Köhler G, Ryffel B, Schaub GA, Mossmann H, Brombacher F (2000) Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun 68(7):4075–4083
Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD (2006) Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol 177:169–176
Gruver AL, Ventevogel MS, Sempowski GD (2009) Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J Endocrinol 203:75–85
Torpy DJ, Bornstein SR, Chrousos GP (1998) Leptin and interleukin-6 in sepsis. Horm Metab Res 30:726–729
van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RH, West CE, van der Meer JW (2002) Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab 87:758–763
Santucci N, D’Attilio L, Kovalevski L, Bozza V, Besedovsky H, del Rey A, Bay ML, Bottasso O (2011) A multifaceted analysis of immune-endocrine-metabolic alterations in patients with pulmonary tuberculosis. PLoS ONE 6:e26363
Militsi H, Lazaropoulou C, Kariyannis C, Karakonstantakis T, Demetriou E, Theodoridou M, Papassotiriou I (2005) Increased leptin levels in children with infections. Pediatr Res 58:403–403. doi:10.1203/00006450-200508000-00310
Marie CS, Verkerke HP, Paul SN, Mackey AJ, Petri WA Jr (2012) Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect Immun 80:1934
Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR (1997) Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med 185:171–175
Gaetke LM, Oz HS, Frederich RC, McClain CJJ (2003) Anti-TNF-alpha antibody normalizes serum leptin in IL-2 deficient mice. Am Coll Nutr 22(5):415–420
Murdoch DR, Rooney E, Dargie HJ, Shapiro D, Morton JJ, Mc-Murray JJ (1999) Inappropriately low plasma leptin concentration in the cachexia associated with chronic heart failure. Heart 82:352–356
Faggioni R, Fuller J, Moser A, Feingold KR, Grunfeld C (1997) LPS induced anorexia in leptin-deficient (ob/ob) and leptin receptor deficient (db/db) mice. Am J Physiol 273:R181–R186
Sanchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C (2003) Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol 133:11–19
Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW (1998) Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes 47:538–543
Sahu A, Nguyen L, O’Doherty RM (2002) Nutritional regulation of hypothalamic leptin receptor gene expression is defective in diet induced obesity. J Neuroendocrinol 14:887–893
Houseknecht KL, Portocarrero CP (1998) Leptin and its receptors: regulators of whole-body energy homeostasis. Domest Anim Endocrinol 15(6):457–475
Dutra SC, de Moura EG, Lisboa PC, Trevenzoli IH, Passos MC (2011) Leptin-programmed rats respond to cold exposure changing hypothalamic leptin receptor and thyroid function differently from cold-exposed controls. Regul Pept 171:58–64
Procaccini C, Jirillo E, Matarese G (2012) Leptin as an immunomodulator. Mol Aspects Med 33:35–45
Dardenne M, Savino W, Gastinel LN, Nabarra B, Bach JF (1983) Thymic dysfunction in the mutant diabetic (db/db) mouse. J Immunol 130:1195–1199
Roggero E, Pérez AR, Tamae-Kakazu M, Piazzon I, Nepomnaschy I, Besedovsky HO, Bottasso OA, del Rey A (2006) Endogenous glucocorticoids cause thymus atrophy but are protective during acute Trypanosoma cruzi infection. J Endocrinol 190(2):495–503
Acknowledgments
S.R.V., A.R.P. and O.A.B. are members of The National Council Research (CONICET). R. M. thanks CONICET for a fellowship. This work was supported by grants from the Secretary of Sciences and Technology of National University of Rosario (SCYT-UNR, 1MED-348), National Agency for Scientific and Technological Promotion (ANPCYT, PICT 2008-0980) and Argentine Federation of Cardiology (FAC-GADOR 2011). The authors thank Mrs. Vanina Tartalini for their technical assistance and Gustavo Capriotti from Wiener Lab. Foundation for their help in supplying some laboratory reagents.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ana Rosa Pérez and Oscar Bottasso contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
430_2013_294_MOESM1_ESM.tif
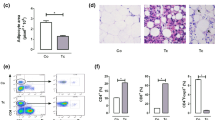
a) Plasma leptin levels in TNF-R1 KO mice compared with wild-type (WT) mice. Values represent the mean ± SEM of 3–5 mice/group/day (one of two independent experiments). Data correspond to results obtained after 14 days post-infection (dpi). b) mRNA expression levels of TNF-α in epididymal fat pads from control and infected animals were analyzed by real-time RT-PCR. The results are expressed as the relative difference to the uninfected control by 2−∆∆CT method. Bars and lines represent the mean ± SEM of 4-6 mice/group. * p < 0.05 vs. the corresponding uninfected group; § p < 0.05 vs. the corresponding uninfected mice; # p < 0.05 vs. 14 dpi; n.d. = non-detectable values. Results are representative of two or three independent experimental rounds, respectively (TIFF 262 kb)
Rights and permissions
About this article
Cite this article
Manarin, R., Villar, S.R., Fernández Bussy, R. et al. Reciprocal influences between leptin and glucocorticoids during acute Trypanosoma cruzi infection. Med Microbiol Immunol 202, 339–352 (2013). https://doi.org/10.1007/s00430-013-0294-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-013-0294-1