Abstract
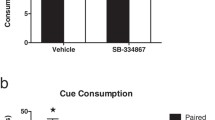
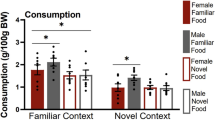
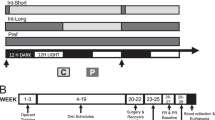
Palatable foods can stimulate appetite without hunger, and unconstrained overeating underlies obesity and binge eating disorder. Women are more prone to obesity and binge eating than men but the neural causes of individual differences are unknown. In an animal model of hedonic eating, a prior study found that females were more susceptible than males to eat palatable food when sated and that the neuropeptide orexin/hypocretin (ORX) was crucial in both sexes. The current study examined potential extra-hypothalamic forebrain targets of ORX signaling during hedonic eating. We measured Fos induction in the cortical, thalamic, striatal, and amygdalar areas that receive substantial ORX inputs and contain their receptors in hungry and sated male and female rats during palatable (high-sucrose) food consumption. During the test, hungry rats of both sexes ate substantial amounts, and while sated males ate much less than hungry rats, sated females ate as much as hungry rats. The Fos induction analysis identified sex differences in recruitment of specific areas of the medial prefrontal cortex, paraventricular nucleus of the thalamus (PVT), nucleus accumbens (ACB), and central nucleus of the amygdala (CEA), and similar patterns across sexes in the insular cortex. There was a striking activation of the infralimbic cortex in sated males, who consumed the least amount food and unique correlations between the insular cortex, PVT, and CEA, as well as the prelimbic cortex, ACB, and CEA in sated females but not sated males. The study identified key functional circuits that may drive hedonic eating in a sex-specific manner.










Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlaping distribution of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237
Barson JR, Leibowitz SF (2015) GABA-induced activation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett 609:92–96
Baumgartner HM, Schulkin J, Berridge KC (2021) Activating corticotropin-releasing factor systems in the nucleus accumbens, amygdala, and bed nucleus of stria terminalis: Incentive motivation or aversive motivation? Biol Psychiatry 89:1162–1175
Boughter JD Jr, Fletcher M (2021) Rethinking the role of taste processing in insular cortex and forebrain circuits. Curr Opin Physio 20:52–56
Buczek L, Migliaccio J, Petrovich GD (2020) Hedonic eating: sex differences and characterization of orexin activation and signaling. Neuroscience 436:34–45
Cai H, Haubensak W, Anthony TE, Anderson DJ (2014) Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 17(17):1240–1248
Capuzzo G, Floresco SB (2020) Prelimbic and infralimbic prefrontal regulation of active and inhibitory avoidance and reward-seeking. J Neurosci 40:4773–4787
Carr KD (2011) Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: binge eating and drug abuse. Physiol Behav 104:162–167
Castro DC, Terry RA, Berridge KC (2016) Orexin in rostral hotspot of nucleus accumbens enhances sucrose ‘liking’ and intake but scopolamine in caudal shell shifts ‘liking’ toward ‘disgust’ and ‘fear.’ Neuropsychopharmacology 41:2101–2111
Choi DL, Davis JF, Fitzerald ME, Benoit SC (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167:11–20
Choi DL, Davis JF, Magrisso IJ, Fitzerald ME, Lipton JW, Benoit SC (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210:243–248
Christoffel DJ, Walsh JJ, Heifets BD, Hoerbelt P, Neuner S, Sun G, Ravikumar VK, Wu H, Halpern CH, Malenka RC (2021) Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nature Comunications 12:2135
Cole S, Hobin MP, Petrovich GD (2015a) Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience 286:187–202
Cole S, Mayer HS, Petrovich GD (2015b) Orexin/hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep 5:16143
Cole S, Keefer SE, Anderson LC, Petrovich GD (2020) Medial prefrontal cortex neural plasticity, orexin receptor 1 signaling, and connectivity with the lateral hypothalamus are necessary in cue-potentiated feeding. J Neurosci 40:1744–1755
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327
Dong X, Li S, Kirouac GJ (2017) Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct Funct 222:3927–3943
Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, Morales PLA, Conzelmann KK, Lüthi A, Klein R (2017) Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci 20:1384–1394
Gao C, Leng Y, Ma J, Rooke V, Rodriguez-Gonzalez S, Ramakrishnan C, Deisseroth K, Penzo MA (2020) Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci 23:217–228
Giacomini JL, Sadeghian K, Baldo BA (2022) Eating driven by the gustatory insula: contrasting regulation by infralimbic vs. prelimbic cortices. Neuropsychopharmacology 47:1358–1366
Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto J, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL (2019) Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron 102:1037–1052
Hartogsveld B, Quaedflieg CWEM, van Ruitenbeek P, Smeets T (2022) Volume and connectivity differences in brain networks associated with cognitive constructs of binge eating. eNeuro 9:1–31
Hudson JI, Hiripi E, Harrison GP, Kessler RC (2007) The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry 61:348–358
Kelley AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27:765–776
Labouèbe G, Boutrel B, Tarussio D, Thorens B (2016) Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci 8:999–1002
Lee EY, Lee HS (2016) Dual projections of single orexin-or CART-immunoreactive, lateral hypothalamic neurons to the paraventricular thalamic nucleus and nucleus accumbens shell in the rat: Light microscopic study. Brain Res 1634:104–118
Li S, Kirouac GJ (2012) Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct 217:257–273
Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303
Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15:6779–6788
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25
Mitchell S, Shaw D (2015) The worldwide epidemic of female obesity. Best Pract Res Clin Obstet Gynaecol 29:289–299
Peciña S, Schulkin J, Berridge KC (2006) Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol 4:8
Petrovich GD (2018) Feeding behavior survival circuit: anticipation & competition. Curr Opin Behav Sci 24:137–142
Petrovich GD (2019) Orexins and control of feeding by learned cues. In: Fadel JR, Burk JA (eds) The orexins/hypocretins system: functional roles and therapeutic potential, 1st edn. Academic Press, pp 85–98
Petrovich GD (2021) The function of paraventricular thalamic circuitry in the adaptive control of feeding behavior. Front Behav Neurosci 15:671096
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015
Raynolds SM, Berridge KC (2008) Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci 11:423–425
Reppucci CJ, Petrovich GD (2012) Learned food-cue stimulates persistent feeding in sated rats. Appetite 59:437–447
Reppucci CJ, Petrovich GD (2016) Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct 221:2937–2962
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585
Small DM (2009) Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes 33:S44–S48
Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17:4434–4440
Sun X, Kroemer NB, Veldhuizen M, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM (2015) Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci 35:7964–7976
Swanson LW (2018) Brain maps 4.0—Structure of the rat brain: an open access atlas with global nervous system nomenclature ontology and flatmaps. J Comp Neurol 526:935–943
Terrill SJ, Subramanian KS, Lan R, Liu CM, Cortella AM, Noble E, Kanoski SE (2020) Nucleus accumbens melanin-concentrating hormone signaling promotes feeding in a sex-specific manner. Neuropharmacology 178:108270
Thorpe AJ, Kotz CM (2005) Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res 1050:156–162
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75
Zahm DS, Brog JS (1992) On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50:751–767
Zhang M, Kelley AE (2000) Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and Fos expression. Neuroscience 99:267–277
Acknowledgements
This work was supported by the National Institutes of Health, NIDDK grant R01DK085721 to GDP. We thank Dr. Ehri Ryu for helpful advice regarding statistical analyses.
Author information
Authors and Affiliations
Contributions
GDP conceptualized the study and supervised the experiments and directed the data analysis. GDP, LB and JM designed the experiments. LB and JM carried out the experiment. WP, EG, LB, JM, EC, AMKM conducted the histological preparation and analyses. WP and EG prepared the figures. WP and GDP wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parsons, W., Greiner, E., Buczek, L. et al. Sex differences in activation of extra-hypothalamic forebrain areas during hedonic eating. Brain Struct Funct 227, 2857–2878 (2022). https://doi.org/10.1007/s00429-022-02580-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02580-0