Abstract
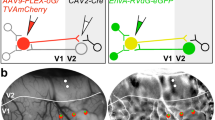
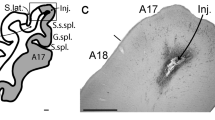
Complementary reciprocal feedforward and feedback circuits connecting the visual thalamus with the visual cortex are essential for visual perception. These circuits predominantly connect primary and secondary visual cortex with the dorsal lateral geniculate nucleus (LGN). Although there are direct geniculocortical inputs to extrastriate visual cortex, whether reciprocal corticogeniculate neurons exist in extrastriate cortex is not known. Here we utilized virus-mediated retrograde tracing to reveal the presence of corticogeniculate neurons in three mid-level extrastriate visual cortical areas in ferrets: PMLS, PLLS, and 21a. We observed corticogeniculate neurons in all three extrastriate areas, although the density of virus-labeled corticogeniculate neurons in extrastriate cortex was an order of magnitude less than that in areas 17 and 18. A cluster analysis of morphological metrics quantified following reconstructions of the full dendritic arborizations of virus-labeled corticogeniculate neurons revealed six distinct cell types. Similar corticogeniculate cell types to those observed in areas 17 and 18 were also observed in PMLS, PLLS, and 21a. However, these unique cell types were not equally distributed across the three extrastriate areas. The majority of corticogeniculate neurons per cluster originated in a single area, suggesting unique parallel organizations for corticogeniculate feedback from each extrastriate area to the LGN. Together, our findings demonstrate direct feedback connections from mid-level extrastriate visual cortex to the LGN, supporting complementary reciprocal circuits at multiple processing stages along the visual hierarchy. Importantly, direct reciprocal connections between the LGN and extrastriate cortex, that bypass V1, could provide a substrate for residual vision following V1 damage.



Similar content being viewed by others
Availability of data, material, and code
Data, material, and custom code that support the findings of this study are available from the corresponding author upon request.
References
Azzopardi P, Fallah M, Gross CG, Rodman HR (2003) Response latencies of neurons in visual areas MT and MST of monkeys with striate cortex lesions. Neuropsychologia 41:1738–1756
Blasdel GG, Lund JS (1983) Termination of afferent axons in macaque striate cortex. J Neurosci 3:1389–1413
Bragg EM, Briggs F (2017) Large-scale reconstructions and independent, unbiased clustering based on morphological metrics to classify neurons in selective populations. J vis Exp 120:1–10
Bragg EM, Fairless EA, Liu S, Briggs F (2017) Morphology of visual sector thalamic reticular neurons in the macaque monkey suggests retinotopically-specialized, parallel stream-mixed input to the lateral geniculate nucleus. J Comp Neurol 525:1273–1290
Briggs F (2020) Role of feedback connections in central visual processing. Annu Rev vis Sci 6:18.1-18.22
Briggs F, Kiley CW, Callaway EM, Usrey WM (2016) Morphological substrates for parallel streams of corticogeniculate feedback originating in both V1 and V2 of the macaque monkey. Neuron 90:388–399
Briggs F, Usrey WM (2005) Temporal properties of feedforward and feedback pathways between thalamus and visual cortex in the ferret. Thalamus Relat Syst 3:133–139
Briggs F, Usrey WM (2007) A fast, reciprocal pathway between the lateral geniculate nucleus and visual cortex in the macaque monkey. J Neurosci 27:5431–5436
Briggs F, Usrey WM (2009) Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron 62:135–146
Calinski T, Harabasz J (1974) A dendrite method for cluster analysis. Commun Stat Theory Methods 3:1–27
Callaway EM (2005) Structure and function of parallel pathways in the primate early visual system. J Physiol 566:13–19
Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J et al (2000) Classification of fusiform neocortical interneurons based on unsupervised clustering. PNAS 97:6144–6149
Conley M, Friederich-Ecsy B (1993) Functional organization of the ventral lateral geniculate complex of the tree shrew (Tupaia belangeri): II. Connections with the cortex, thalamus, and brainstem. J Comp Neurol 328:21–42
Conley M, Raczkowski D (1990) Sublaminar organization within layer 6 of the striate cortex in galago. J Comp Neurol 302:425–436
Cowey A (2010) Visual system: how does blindsight arise? Curr Biol 20:R702–R704
Dell L-A, Innocenti GM, Hilgetag CC, Manger PR (2018) Cortical and thalamic connectivity of occipital visual cortical areas 17, 18, 19, and 21 of the domestic ferret (Mustela putorius furo). J Comp Neurol 527:1293–1314
Dreher B, Michalski A, Ho RH, Lee CW, Burke W (1993) Processing of form and motion in area 21a of cat visual cortex. Vis Neurosci 10:93–115
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Fitzgibbon T, Bittar RG, Dreher B (1999) Projections from striate and extrastriate visual cortices of the cat to the reticular thalamic nucleus. J Comp Neurol 410:467–488
Fitzpatrick D, Usrey WM, Schofield BR, Einstein G (1994) The sublaminar organization of corticogeniculate neurons in layer 6 of macaque striate cortex. Visual Neurosci 11:307–315
Gilbert CD, Kelly JP (1975) The projections of cells in different layers of the cat’s visual cortex. J Comp Neurol 163:81–106
Girard P, Salin PA, Bullier J (1992) Response selectivity of neurons in area MT of the macaque monkey during reversible inactivation of area V1. J Neurophysiol 67:1437–1446
Grant S, Hilgetag CC (2005) Graded classes of cortical connections: quantitative analyses of laminar projections to motion areas of cat extrastriate cortex. Eur J Neurosci 22:681–696
Grieve KL, Sillito AM (1995) Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J Neurosci 15:4868–4874
Harvey AR (1978) Characteristics of corticothalamic neurons in area 17 of the cat. Neurosci Lett 7:177–181
Hasse JM, Bragg EM, Murphy AJ, Briggs F (2019) Morphological heterogeneity among corticogeniculate neurons in ferrets: quantification and comparison with a previous report in macaque monkeys. J Comp Neurol 527:546–557
Hasse JM, Briggs F (2017a) Corticogeniculate feedback sharpens the temporal precision and spatial resolution of visual signals in the ferret. Proc Natl Acad Sci USA 114:E6222–E6230
Hasse JM, Briggs F (2017b) A cross-species comparison of corticogeniculate structure and function. Vis Neurosci 34:1–9
Hendrickson AE, Wilson JR, Ogren MP (1978) The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in the old world and new world primates. J Comp Neurol 182:123–136
Homman-Ludiye J, Manger PR, Bourne JA (2010) Immunohistochemical parcellation of the ferret (Mustela putorius) visual cortex reveals substantial homology with the cat (Felis catus). J Comp Neurol 518:4439–4462
Humphrey AL, Sur M, Uhlrich DJ, Sherman SM (1985a) Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol 233:159–189
Humphrey AL, Sur M, Uhlrich DJ, Sherman SM (1985b) Termination patterns of individual X- and Y-cell axons in the visual cortex of the cat: projections to area 18, to the 17/18 border region, and to both areas 17 and 18. J Comp Neurol 233:190–212
Hupfeld D, Distler C, Hoffmann KP (2007) Deficits of visual motion perception and optokinetic nystagmus after posterior suprasylvian lesions in the ferret (Mustela putorius furo). Exp Brain Res 182:509–523
Ichida JM, Mavity-Hudson JA, Casagrande VA (2014) Distinct patterns of corticogeniculate feedback to different layers of the lateral geniculate nucleus. Eye Brain 6:57–73
Jackson CA, Hickey TL (1985) Use of ferrets in studies of the visual system. Lab Anim Sci 35:211–215
Jarosiewicz B, Schummers J, Malik WQ, Brown EN, Sur M (2012) Functional biases in visual cortex neurons with identified projections to higher cortical targets. Curr Biol 22:269–277
Jayakumar J, Roy S, Dreher B, Martin PR, Vidyasagar TR (2013) Multiple pathways carry signals from short-wavelength-sensitive (‘blue’) cones to the middle temporal area of the macaque. J Physiol 591:339–352
Jones EG (2002) Thalamic organization and function after Cajal. Prog Brain Res 136:333–357
Kaplan E (2004) The M, P, and K pathways of the primate visual system. In: Chalupa L, Werner J (eds) The Visual Neurosciences. MIT Press, Cambridge, pp 481–493
Katz LC (1987) Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci 7:1223–1249
Lempel AA, Nielsen KJ. 2019. Ferrets as a Model for Higher-Level Visual Motion Processing. Curr Biol 29: 179–91 e5
Li B, Li BW, Chen Y, Wang LH, Diao YC (2000) Response properties of PMLS and PLLS neurons to simulated optic flow patterns. Eur J Neurosci 12:1534–1544
Lin CS, Kaas JH (1977) Projections from cortical visual areas 17, 18, and MT onto the dorsal lateral geniculate nucleus in owl monkeys. J Comp Neurol 173:457–474
Lund JS, Boothe RG (1975) Interlaminar connections and pyramidal neuron organization in the visual cortex, area 17, of the macaque monkey. J Comp Neurol 159:305–334
Lund JS, Lund RD, Hendrickson AE, Bunt AH, Fuchs AF (1975) The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol 164(287):303
Lyon DC, Rabideau C (2012) Lack of robust LGN label following transneuronal rabies virus injections into macaque area V4. J Comp Neurol 520:2500–2511
Lysakowski A, Standage GP, Benevento LA (1988) An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study. Exp Brain Res 69:651–661
Michalski A, Wimborne BM, Henry GH (1993) The effect of reversible cooling of cat’s primary visual cortex on the responses of area 21a neurons. J Physiol 466:133–156
Osakada F, Mori T, Cetin A, Marshel JH, Virgen B, Callaway EM (2011) New rabies virus varients for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71:617–631
Philipp R, Distler C, Hoffmann KP (2006) A motion-sensitive area in ferret extrastriate visual cortex: an analysis in pigmented and albino animals. Cereb Cortex 16:779–790
Radtke-Schuller S (2018) Cyto- and myeloarchitectural brain atlas of the ferret (Mustela putorius) in MRI aided stereotaxic coordinates. Springer Press, New York
Rockland KS (1994) Further evidence for two types of corticopulvinar neurons. NeuroReports 5(1865):68
Rodman HR, Gross CG, Albright TD (1989) Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal. J Neurosci 9:2033–2050
Rosa MGP, Tweedale R, Elston GN (2000) Visual responses of neurons in the middle temporal area of new world monkeys after lesions of striate cortex. J Neurosci 20:5552–5563
Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M et al (2010) Blindsight depends on the lateral geniculate nucleus. Nature
Sherk H (1986) Coincidence of patchy inupts from the lateral geniculate complex and area 17 to the cat’s Clare-Bishop area. J Comp Neurol 253:105–120
Sherman SM, Guillery RW (2006) Exploring the thalamus and its role in cortical function. MIT Press, Boston
Sincich LC, Park KF, Wohlgemuth MJ, Horton JC (2004) Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 7:1123–1128
Talebi V, Baker CI (2016) Categorically distinct types of receptive fields in early visual cortex. J Neurophysiol 115:2556–2576
Thorndike RL (1953) Who belongs in the family? Psychometrika 18:267–276
Tong L, Kalil RE, Spear PD (1982) Thalamic projections to visual areas of the middle suprasylvian sulcus in the cat. J Comp Neurol 212:103–117
Tsumoto T, Suda K (1980) Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. J Comp Neurol 193:223–236
Usrey WM, Fitzpatrick D (1996) Specificity in the axonal connections of layer 6 neurons in tree shrew striate cortex: evidence for distinct granular and supragranular systems. J Neurosci 16:1203–1218
Wang C, Waleszczyk WJ, Burke W, Dreher B (2000) Modulatory influence of feedback projections from area 21a on neuronal activities in striate cortex of the cat. Cereb Cortex 10:1217–1232
Weiskrantz L (2009) Is blindsight just degraded normal vision? Exp Brain Res 192:413–416
Wickersham IR, Finke S, Conzelmann KK, Callaway EM (2007) Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4:47–49
Acknowledgements
We thank Elise Bragg for expert technical assistance and Drs. Karen Moodie and Kirk Maurer for veterinary assistance. We thank Allison Murphy for helpful comments on the manuscript. Note: Invited submission to Brain Structure and Function special issue on “Structure and Function of the Visual System” edited by Drs. Takemura and Rosa.
Funding
This work was funded by National Institutes of Health (National Eye Institute: EY018683 and EY025219 to F.B.), National Science Foundation (EPSCoR 1632738), the Whitehall Foundation, the Hitchcock Foundation, the Del Monte Institute for Neuroscience at the University of Rochester, and a University of Rochester University Research Award. J.M.H. was supported by a Graduate Fellowship from the Albert J. Ryan Foundation.
Author information
Authors and Affiliations
Contributions
M.A. and F.B. designed the study. J.M.H. and F.B. collected the data. J.M.H. performed histological processing. M.A. analyzed the data. M.A. and F.B. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adusei, M., Hasse, J.M. & Briggs, F. Morphological evidence for multiple distinct channels of corticogeniculate feedback originating in mid-level extrastriate visual areas of the ferret. Brain Struct Funct 226, 2777–2791 (2021). https://doi.org/10.1007/s00429-021-02385-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02385-7