Abstract
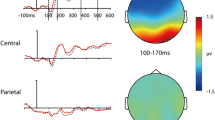
Metacontrast masking is a powerful illusion to investigate the dynamics of perceptual processing and to control conscious visual perception. However, the neural mechanisms underlying this fundamental investigative tool are still debated. In the present study, we examined metacontrast masking across different contrast polarities by employing a contour discrimination task combined with EEG (Electroencephalography). When the target and mask had the same contrast polarity, a typical U-shaped metacontrast function was observed. A change in mask polarity (i.e., opposite mask polarity) shifted this masking function to a monotonic increasing function such that the target visibility was strongly suppressed at stimulus onset asynchronies less than 50 ms. This transition in metacontrast function has been typically interpreted as an increase in intrachannel inhibition of the sustained activities functionally linked to object visibility and identity. Our EEG analyses revealed an early (160–300 ms) and a late (300–550 ms) spatiotemporal cluster associated with this effect of polarity. The early cluster was mainly over occipital and parieto-occipital scalp sites. On the other hand, the later modulations of the evoked activities were centered over parietal and centro-parietal sites. Since both of these clusters were beyond 160 ms, the EEG results point to late recurrent inhibitory mechanisms. Although the findings here do not directly preclude other proposed mechanisms for metacontrast, they highlight the involvement of recurrent intrachannel inhibition in metacontrast masking.







Similar content being viewed by others
Data availability
The dataset is available from the corresponding author on request. Any access to the data will be granted in accordance with the informed consent signed by the participants.
References
Akyuz S, Pavan A, Kaya U, Kafaligonul H (2020) Short- and long-term forms of neural adaptation: an ERP investigation of dynamic motion aftereffects. Cortex 125:122–134
Bachmann T (1988) Time course of the subjective contrast enhancement for a second stimulus in successively paired above-threshold transient forms: perceptual retouch instead of forward masking. Vis Res 28:1255–1261
Bachmann T (1994) Psychophysiology of visual masking: the fine structure of conscious experience. Nova Science Publishers, Commack, NY
Bachmann T, Francis G (2013) Visual masking: studying perception, attention, and consciousness. Academic Press, Oxford, UK
Brainard D (1997) The psychophysics toolbox. Spat Vis 10:433–436
Breitmeyer BG (1978a) Metacontrast with black and white stimuli: evidence of inhibition of on and off sustained activity by either on or off transient activity. Vis Res 18:1443–1448
Breitmeyer BG (1978b) Metacontrast masking as a function of mask energy. Bull Psychon Soc 12:50–52
Breitmeyer BG, Ogmen H (2000) Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys 62:1572–1595
Breitmeyer BG, Ogmen H (2006) Visual masking: time slices through conscious and unconscious vision, 2nd edn. Oxford University Press, Oxford, UK
Breitmeyer BG, Ogmen H, Chen J (2004) Unconscious priming by color and form: different processes and levels. Conscious Cogn 13:138–157
Breitmeyer BG, Kafalıgönül H, Öğmen H, Mardon L, Todd S, Ziegler R (2006) Meta- and paracontrast reveal differences between contour and brightness processing mechanisms. Vis Res 46:2645–2658
Breitmeyer BG, Tapia E, Kafalıgönül H, Öğmen H (2008) Metacontrast masking and stimulus contrast polarity. Vis Res 48:2433–2438
Bridgeman B (1988) Visual evoked potentials: concomitants of metacontrast in late components. Percept Psychophys 43:401–403
Cecere R, Gross J, Willis A, Thut G (2017) Being first matters: topographical representational similarity analysis of ERP signals reveals separate networks for audiovisual temporal binding depending on the leading sense. J Neurosci 37(21):5274–5287
Del Cul A, Baillet S, Dehaene S (2007) Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol 5:2408–2423
Donchin E, Wicke JD, Lindsley DB (1963) Cortical evoked potentials and perception of paired flashes. Science 141(3587):1285–1286
Dow BM (1974) Functional classes of cells and their laminar distribution in monkey visual cortex. J Neurophysiol 37:927–946
Fahrenfort JJ, Scholte HS, Lamme VAF (2007) Masking disrupts reentrant processing in human visual cortex. J Cogn Neurosci 19:1488–1497
Förster J, Koivisto M, Revonsuo A (2020) ERP and MEG correlates of visual consciousness: the second decade. Conscious Cogn 80:102917
Francis G (2000) Quantitative theories of metacontrast masking. Psychol Rev 107:768–785
Growney R, Weisstein N (1972) Spatial characteristics of metacontrast. J Opt Soc Am 62(5):690–696
Haynes J-D, Driver J, Rees G (2005) Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron 46:811–821
Hubel DH, Wiesel TN (1968) Receptive fields and functional architecture of monkey striate cortex. J Physiol 195:215–243
Jansen M, Jin J, Li X, Lashgari R, Kremkow J, Bereshpolova Y, Swadlow HA, Zaidi Q, Alonso JM (2019) Cortical balance between ON and OFF visual responses is modulated by the spatial properties of the visual stimulus. Cereb Cortex 29(1):336–355
Jeffreys DA, Musselwhite MJ (1986) A visual evoked potential study of metacontrast masking. Vis Res 26:631–642
Kafaligönül H, Breitmeyer BG, Öğmen H (2009) Effects of contrast polarity in paracontrast masking. Atten Percept Psychophys 71(7):1576–1587
Kafaligonul H, Breitmeyer BG, Öğmen H (2015) Feedforward and feedback processes in vision. Front Psychol 6:279
Kaplan E, Shapley RM (1986) The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci USA 83(8):2755–2757
Kaya U, Kafaligonul H (2019) Cortical processes underlying the effects of static sound timing on perceived visual speed. Neuroimage 199:194–205
Kleiner M, Brainard D, Pelli D (2007) What’s new in Psychtoolbox-3? Perception 36(14):1–16
Koivisto M, Grassini S (2016) Neural processing around 200 ms after stimulus-onset correlates with subjective visual awareness. Neuropsychologia 84:235–243
Koivisto M, Revonsuo A (2010) Event-related brain potential correlates of visual awareness. Neurosci Biobehav Rev 34:922–934
Komban SJ, Kremkow J, Jin J, Wang Y, Lashgari R, Li X, Zaidi Q, Alonso JM (2014) Neuronal and perceptual differences in the temporal processing of darks and lights. Neuron 82:224–234
Lamme VAF, Roelfsema PR (2000) The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci 23:571–579
Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164(1):177–190
Norcia AM, Yakovleva A, Hung B, Goldberg JL (2020) Dynamics of contrast decrement and increment responses in human visual cortex. Transl Vis Sci Technol 9(10):6
Ogmen H, Breitmeyer BG, Melvin R (2003) The what and where in visual masking. Vis Res 43:1337–1350
Oluk C, Pavan A, Kafaligonul H (2016) Rapid motion adaptation reveals the temporal dynamics of spatiotemporal correlation between ON and OFF pathways. Sci Rep 6:34073
Pelli D (1997) The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10:437–442
Pitts MA, Padwal J, Fennelly D, Martínez A, Hillyard SA (2014) Gamma band activity and the P3 reflect post-perceptual processes, not visual awareness. Neuroimage 101:337–350
Railo H, Koivisto M (2009) The electrophysiological correlates of stimulus visibility and metacontrast masking. Conscious Cogn 18:794–803
Rieger JW, Braun C, Bülthoff HH, Gegenfurtner KR (2005) The dynamics of visual pattern masking in natural scene processing: a magnetoencephalography study. J Vis 5(3):275–286
Roveri L, Demarco PJ, Celesia GG (1997) An electrophysiological metric of activity within the ON- and OFF-pathways in humans. Vis Res 37:669–674
Schiller PH (1982) Central connections to the ON- and OFF-pathways. Nature 297:1288–1374
Schiller PH (1992) The ON and OFF channels of the visual system. Trends Neurosci 15:86–92
Schiller P, Chorover L (1966) Metacontrast: its relation to evoked potentials. Science 153:1398–1400
Schiller PH, Finlay BL, Volman SF (1976) Quantitative studies of single cell properties in monkey striate cortex. I–V. J Neurophysiol 39:1288–1374
Sherrick MF, Keating JK, Dember WN (1974) Metacontrast with black and white stimuli. Can J Psychol 28:438–445
Sterkin A, Yehezkel O, Bonneh YS, Norcia A, Polat U (2009) Backward masking suppresses collinear facilitation in the visual cortex. Vis Res 49:1784–1794
Stewart AL, Purcell DG (1974) Visual backward masking by a flash of light: a study of u-shaped detection functions. J Exp Psychol 103(3):553–566
Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM (2011) Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011:e879716
Thaler L, Schütz AC, Goodale MA, Gegenfurtner KR (2013) What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vis Res 76:31–42
Uusitalo MA, Ilmoniemi RJ (1997) Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35(2):135–140
Van Aalderen-Smeets SI, Oostenveld R, Schwarzbach J (2006) Investigating neurophysiological correlates of metacontrast masking with magnetoencephalography. Adv Cogn Psychol 2:21–35
Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL (1964) Contingent negative variation: an electric sign of sensori-motor association and expectancy in the human brain. Nature 203(4943):380–384
World Medical Association (2013) Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Wutz A, Melcher D, Samaha J (2018) Frequency modulation of neural oscillations according to visual task demands. Proc Natl Acad Sci USA 115:1346–1351
Zemon V, Gordon J (2006) Luminance-contrast mechanisms in humans: visual evoked potentials and a nonlinear model. Vis Res 46:4163–4180
Zemon V, Gordon J, Welch J (1988) Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Vis Neurosci 1:145–150
Acknowledgements
We would like to thank Utku Kaya for suggestions on the data analyses. We are also grateful to the anonymous reviewers whose detailed and helpful comments significantly improved our manuscript.
Funding
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK, Grant Number 119K368).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no actual or potential conflicts of interest.
Ethical approval
All experimental procedures were in accordance with the Declaration of Helsinki and international guidelines and approved by the local ethics committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aydin, A., Ogmen, H. & Kafaligonul, H. Neural correlates of metacontrast masking across different contrast polarities. Brain Struct Funct 226, 3067–3081 (2021). https://doi.org/10.1007/s00429-021-02260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02260-5