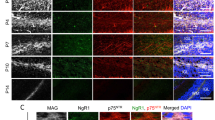
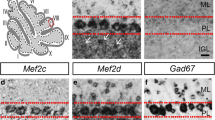
Abstract
Neurofibromatosis type 2 (NF2) patients are prone to develop glial-derived tumors in the peripheral and central nervous system (CNS). The Nf2 gene product –Merlin is not only expressed in glia, but also in neurons of the CNS, where its function still remains elusive. Here, we show that cerebellar Purkinje cells (PCs) of isoform-specific Merlin-deficient mice were innervated by smaller vGluT2-positive clusters at presynaptic terminals than those of wild-type mice. This was paralleled by a reduction in frequency and amplitude of miniature excitatory postsynaptic currents (mEPSC). On the contrary, in conditional transgenic mice in which Merlin expression was specifically ablated in PCs (L7Cre;Nf2fl/fl), we found enlarged vGluT2-positive clusters in their presynaptic buttons together with increased amplitudes of miniature postsynaptic currents. The presynaptic terminals of these PCs innervating neurons of the deep cerebellar nuclei were also enlarged. When exploring mice with Merlin-deficient granule cells (GCs) (Math1Cre;Nf2fl/fl), we found cerebellar extracts to contain higher amounts of vGluT1 present in parallel fiber terminals. In parallel, mEPSC frequency was increased in Math1Cre;Nf2fl/fl mice. On the contrary, VGluT2 clusters in cerebellar glomeruli composed of NF2-deficient presynaptic Mossy fiber terminals and NF2-deficient postsynaptic GC were reduced in size as shown for isoform-specific knockout mice. These changes in Math1Cre;Nf2fl/fl-deficient mice were paralleled by an increased activation of Rac1–Cofilin signaling which is known to impact on cytoskeletal reorganization and synapse formation. Consistent with the observed synaptic alterations in these transgenic mice, we observed altered ultrasonic vocalization, which is known to rely on proper cerebellar function. No gross morphological changes or motor coordination deficits were observed in any of these transgenic mice. We therefore conclude that Merlin does not regulate overall cerebellar development, but impacts on pre- and post-synaptic terminal organization.










Similar content being viewed by others
References
Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805–809
Avery AW, Thomas DD, Hays TS (2017) β-III-spectrin spinocerebellar ataxia type 5 mutation reveals a dominant cytoskeletal mechanism that underlies dendritic arborization. Proc Natl Acad Sci USA 114:E9376–E9385
Baader SL, Schilling K (1996) Glutamate receptors mediate dynamic regulation of nitric oxide synthase expression in cerebellar granule cells. J Neurosci 16:1440–1449
Bahjaoui-Bouhaddi M, Padilla F, Nicolet M, Cifuentes-Diaz C, Fellmann D, Mege RM (1997) Localized deposition of m-cadherin in the glomeruli of the granular layer during the postnatal development of mouse cerebellum. J Comp Neurol 378:180–195
Bamburg JR (1999) Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15:185–230
Baptista CA, Hatten ME, Blazeski R, Mason CA (1994) Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron 12:243–260
Barski JJ, Dethleffsen K, Meyer M (2000) Cre recombinase expression in cerebellar Purkinje cells. Genesis 28:93–98
Bertling E, Hotulainen P (2017) New waves in dendritic spine actin cytoskeleton. From branches and bundles to rings, from actin binding proteins to post-translational modifications. Mol Cell Neurosci 84:77–84
Borovac J, Bosch M, Okamoto K (2018) Regulation of actin dynamics during structural plasticity of dendritic spines. Signaling messengers and actin-binding proteins. Mol Cell Neurosci 91:122–130
Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD (2010) Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1:15
Chen LY, Rex CS, Casale MS, Gall CM, Lynch G (2007) Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci 27:5363–5372
Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, Lauterborn JC (2010) Physiological activation of synaptic RacPAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci 30:10977–10984
Chiasson-MacKenzie C, Morris ZS, Baca Q, Morris B, Coker JK, Mirchev R, Jensen AE, Carey T, Stott SL, Golan DE, McClatchey AI (2015) NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin. J Cell Biol 211:391–405
Chih B, Engelman H, Scheiffele P (2005) Control of excitatory and inhibitory synapse formation by neuroligins. Science 307:1324–1328
Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC (2007) Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54:919–931
Coesmans M, Weber JT, de Zeeuw CI, Hansel C (2004) Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44:691–700
den Bakker MA, Vissers KJ, Molijn AC, Kros JM, Zwarthoff EC, van der Kwast TH (1999) Expression of the neurofibromatosis type 2 gene in human tissues. J Histochem Cytochem 47:1471–1480
Development Core Team R (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ferner RE (2007) Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol 6:340–351
Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, Sjostrom PJ, van Meyel DJ (2014) Neuronal morphometry directly from bitmap images. Nat Meth 11:982–984
Fujita E, Momoi T (2014) Specific expression of FOXP2 in cerebellum improves ultrasonic vocalization in heterozygous but not in homozygous Foxp2 (R552H) knock-in pups. Neurosci Lett 566:162–166
Georges PC, Hadzimichalis NM, Sweet ES, Firestein BL (2008) The yin–yang of dendrite morphology: unity of actin and microtubules. Mol Neurobiol 38:270–284
Gliem M, Weisheit G, Mertz KD, Endl E, Oberdick J, Schilling K (2006) Expression of classical cadherins in the cerebellar anlage: quantitative and functional aspects. Mol Cell Neurosci 33:447–458
Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ (2013) Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 19:337–344
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119:1013–1026
Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ (2010) ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci 13:1208–1215
Gutmann DH, Wright DE, Geist RT, Snider WD (1995) Expression of the neurofibromatosis 2 (NF2) gene isoforms during rat embryonic development. Hum. Mol. Genet. 4:471–478
Hansen ST, Meera P, Otis TS, Pulst SM (2012) Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet 22:271–283
Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M (2009) Translocation of a “Winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “Losers” from the soma in developing cerebellum. Neuron 63:106–118
Hayashi ML, Choi S-Y, Rao BSS, Jung H-Y, Lee H-K, Zhang D, Chattarji S, Kirkwood A, Tonegawa S (2004) Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron 42:773–787
Heintz TG, Eva R, Fawcett JW (2016) Regional regulation of Purkinje cell dendritic spines by integrins and Eph/Ephrins. PLoS One 11:e0158558
Hendzel MJ, Wei Y, Mancini MA, van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360
Hennigan RF, Moon CA, Parysek LM, Monk KR, Morfini G, Berth S, Brady S, Ratner N (2013) The NF2 tumor suppressor regulates microtubule-based vesicle trafficking via a novel Rac, MLK and p38(SAPK) pathway. Oncogene 32:1135–1143
Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N, Kaneko T (2003) Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience 117:1–6
Holst MI, Maercker C, Pintea B, Masseroli M, Liebig C, Jankowski J, Miething A, Martini J, Schwaller B, Oberdick J, Schilling K, Baader SL (2008) Engrailed-2 regulates genes related to vesicle formation and transport in cerebellar Purkinje cells. Mol Cell Neurosci 38:495–504
Huynh DP, Tran TM, Nechiporuk T, Pulst SM (1996) Expression of neurofibromatosis 2 transcript and gene product during mouse fetal development. Cell Growth Differ 7:1551–1561
Ichikawa R, Hashimoto K, Miyazaki T, Uchigashima M, Yamasaki M, Aiba A, Kano M, Watanabe M (2016) Territories of heterologous inputs onto Purkinje cell dendrites are segregated by mGluR1-dependent parallel fiber synapse elimination. Proc Natl Acad Sci USA 113:2282–2287
Ito M (2002) The molecular organization of cerebellar long-term depression. Nat Rev Neurosci 3:896–902
Jakab RL, Hámori J (1988) Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anat Embryol 179:81–88
Jankowski J, Holst MI, Liebig C, Oberdick J, Baader SL (2004) Engrailed-2 negatively regulates the onset of perinatal Purkinje cell differentiation. J Comp Neurol 472:87–99
Jannatipour M, Dion P, Khan S, Jindal H, Fan X, Laganiere J, Chishti AH, Rouleau GA (2001) Schwannomin isoform-1 interacts with syntenin via PDZ domains. J Biol Chem 276:33093–33100
Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, Scheiffele P (2011) Development of axon-target specificity of ponto-cerebellar afferents. PLoS Biol 9:e1001013
Kissil JL, Johnson KC, Eckman MS, Jacks T (2002) Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 277:10394–10399
Kuhn TB, Meberg PJ, Brown MD, Bernstein BW, Minamide LS, Jensen JR, Okada K, Soda EA, Bamburg JR (2000) Regulating actin dynamics in neuronal growth cones by ADF/cofilin and Rho family GTPases. J Neurobiol 44:126–144
Li W, Cooper J, Karajannis MA, Giancotti FG (2012) Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep 13(3):204–215
Liu A, Zhou Z, Dang R, Zhu Y, Qi J, He G, Leung C, Pak D, Jia Z, Xie W (2016) Neuroligin 1 regulates spines and synaptic plasticity via LIMK1/cofilin-mediated actin reorganization. J Cell Biol 212:449–463
Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B (2005) Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn 234:633–650
McClatchey AI, Fehon RG (2009) Merlin and the ERM proteins–regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 19:198–206
McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T (1997) The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev 11:1253–1265
Meng Y, Takahashi H, Meng J, Zhang Y, Lu G, Asrar S, Nakamura T, Jia Z (2004) Regulation of ADF/cofilin phosphorylation and synaptic function by LIM-kinase. Neuropharmacology 47:746–754
Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P (2007) Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res 67:520–527
Muller W, Connor JA (1991) Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature 354:73–76
Nam CI, Chen L (2005) Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci USA 102:6137–6142
Ng J, Luo L (2004) Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 44:779–793
Palay SL, Chan-Palay V (1974) Cerebellar cortex. Cytology and organization. Springer, New York
Pan N, Jahan I, Lee JE, Fritzsch B (2009) Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res 337:407–428
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME (1997) Development of polarity in cerebellar granule cells. J Neurobiol 32:223–236
Racz B, Weinberg RJ (2006) Spatial organization of cofilin in dendritic spines. Neuroscience 138:447–456
Ramesh V (2004) Merlin and the ERM proteins in Schwann cells, neurons and growth cones. Nat Rev Neurosci 5:462–470
Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16:522–529
Roszkowska M, Skupien A, Wojtowicz T, Konopka A, Gorlewicz A, Kisiel M, Bekisz M, Ruszczycki B, Dolezyczek H, Rejmak E, Knapska E, Mozrzymas JW, Wlodarczyk J, Wilczynski GM, Dzwonek J (2016) CD44: a novel synaptic cell adhesion molecule regulating structural and functional plasticity of dendritic spines. Mol Biol Cell 27:4055–4066
Sarowar T, Grabrucker AM (2016) Actin-dependent alterations of dendritic spine morphology in shankopathies. Neural Plast 2016:8051861
Sarowar T, Grabrucker S, Föhr K, Mangus K, Eckert M, Bockmann J, Boeckers TM, Grabrucker AM (2016) Enlarged dendritic spines and pronounced neophobia in mice lacking the PSD protein RICH2. Mol Brain 9:409
Sassoe-Pognetto M, Patrizi A (2017) The Purkinje cell as a model of synaptogenesis and synaptic specificity. Brain Res Bull 129:12–17
Scattoni ML, Gandhy SU, Ricceri L, Crawley JN (2008) Unusual repertoire of vocalizations in the BTBR T + tf/J mouse model of Autism. PLoS One 3:e3067–e3081
Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101:657–669
Schilling K, Dickinson MH, Connor JA, Morgan JI (1991) Electrical activity in cerebellar cultures determines Purkinje cell dendritic growth patterns. Neuron 7:891–902
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Meth 9:676–682
Schulz A, Geissler KJ, Kumar S, Leichsenring G, Morrison H, Baader SL (2010) Merlin inhibits neurite outgrowth in the CNS. J Neurosci 30:10177–10186
Schulz A, Baader SL, Niwa-Kawakita M, Jung MJ, Bauer R, Garcia CA, Zoch A, Schacke S, Hagel C, Mautner VF, Hanemann CO, Dun XP, Parkinson DB, Weis J, Schroder JM, Gutmann DH, Giovannini M, Morrison H (2013a) Merlin isoform 2 in neurofibromatosis type 2-associated polyneuropathy. Nat Neurosci 16:426–433
Schulz A, Kyselyova A, Baader SL, Jung MJ, Zoch A, Mautner VF, Hagel C, Morrison H (2013b) Neuronal merlin influences ERBB2 receptor expression on Schwann cells through neuregulin 1 type III signalling. Brain 137:420–432
Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O’Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI (2001) The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 1:63–72
Sher I, Hanemann CO, Karplus PA, Bretscher A (2012) The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell 22:703–705
Shevelkin AV, Ihenatu C, Pletnikov MV (2014) Pre-clinical models of neurodevelopmental disorders: focus on the cerebellum. Rev Neurosci 25:177–194
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387–406
Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, de Gasperi R, Sosa MAG, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD (2005) Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA 102:9643–9648
Sotelo C, Dusart I (2009) Intrinsic versus extrinsic determinants during the development of Purkinje cell dendrites. Neuroscience 162:589–600
Surace EI, Haipek CA, Gutmann DH (2004) Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene 23:580–587
Threadgill R, Bobb K, Ghosh A (1997) Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19:625–634
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M (2012) Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488:647–651
Unda BK, Kwan V, Singh KK (2016) Neuregulin-1 regulates cortical inhibitory neuron dendrite and synapse growth through DISC1. Neural Plast 2016:7694385
Voogd J, Glickstein M (1998) The anatomy of the cerebellum. Trends Neurosci 21:370–375
White JJ, Arancillo M, Stay TL, George-Jones NA, Levy SL, Heck DH, Sillitoe RV (2014) Cerebellar zonal patterning relies on Purkinje cell neurotransmission. J Neurosci 34:8231–8245
Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR (2000) Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav 37:145–155
Witter L, Rudolph S, Pressler RT, Lahlaf SI, Regehr WG (2016) Purkinje cell collaterals enable output signals from the cerebellar cortex to feed back to purkinje cells and interneurons. Neuron 91:312–319
Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN (2011) Communication impairments in mice lacking Shank1. Reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One 6:e20631
Wolf M, Zimmermann A-M, Görlich A, Gurniak CB, Sassoè-Pognetto M, Friauf E, Witke W, Rust MB (2015) ADF/Cofilin controls synaptic actin dynamics and regulates synaptic vesicle mobilization and exocytosis. Cereb Cortex 25:2863–2875
Wood KA, Dipasquale B, Youle RJ (1993) Insitu labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron 11:621–632
Xing W, Li M, Zhang F, Ma X, Long J, Zhou H (2017) The conformation change and tumor suppressor role of Merlin are both independent of Serine 518 phosphorylation. Biochem Biophys Res Commun 493:46–51
Yamauchi J, Miyamoto Y, Kusakawa S, Torii T, Mizutani R, Sanbe A, Nakajima H, Kiyokawa N, Tanoue A (2008) Neurofibromatosis 2 tumor suppressor, the gene induced by valproic acid, mediates neurite outgrowth through interaction with paxillin. Exp Cell Res 314:2279–2288
Zhou Q, Homma KJ, Poo MM (2004) Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44:749–757
Zoch A, Mayerl S, Schulz A, Greither T, Frappart L, Rubsam J, Heuer H, Giovannini M, Morrison H (2015) Merlin isoforms 1 and 2 both act as tumour suppressors and are required for optimal sperm maturation. PLoS One 10:e0129151
Acknowledgements
This work was supported by the Alexander von Humboldt foundation (HERMES to A.T.) and BONFOR grant O-167.0017 (to S.L.B.). The authors are very grateful to Stefanie Ramrath and Sabine Molly-Klumbies for excellent technical help, and Daniela Krauss and Narziss Haias for provident animal husbandry. We would like to acknowledge Dr. Daniel Prieto for thorough comments during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest, neither commercial nor non-commercial. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (see “Materials and methods” section for details). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toledo, A., Lang, F., Doengi, M. et al. Merlin modulates process outgrowth and synaptogenesis in the cerebellum. Brain Struct Funct 224, 2121–2142 (2019). https://doi.org/10.1007/s00429-019-01897-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-019-01897-7