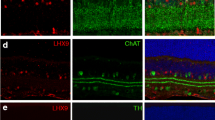
Abstract
The amino acid glycine acts as a neurotransmitter at both inhibitory glycinergic and excitatory glutamatergic synapses predominantly in caudal regions of the central nervous system but also in frontal brain regions and the retina. After its presynaptic release and binding to postsynaptic receptors at caudal glycinergic synapses, two high-affinity glycine transporters GlyT1 and GlyT2 remove glycine from the extracellular space. Glycinergic neurons express GlyT2, which is essential for the presynaptic replenishment of the transmitter, while glial-expressed GlyT1 was shown to control the extracellular glycine concentration. Here we show that GlyT1 expressed by glycinergic amacrine cells of the retina does not only contribute to the control of the extracellular glycine concentration in the retina but is also essential for the maintenance of the glycinergic transmitter phenotype of this cell population. Specifically, loss of GlyT1 from the glycinergic AII amacrine cells impairs AII-mediated glycinergic neurotransmission and alters regulation of the extracellular glycine concentration, without changes in the overall distribution and/or size of glycinergic synapses. Taken together, our results suggest that GlyT1 expressed by amacrine cells in the retina combines functions covered by neuronal GlyT2 and glial GlyT1 at caudal glycinergic synapses.







Similar content being viewed by others
References
Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D et al (2001) ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol Dec 60:1414–1420
Cubelos B, Gimenez C, Zafra F (2005) Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex 15:448–459
Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL (2002) Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36:703–712
Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstätter JH (2001) Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: comparison with Bassoon. J Comp Neurol 439:224–234
Dingledine R, Kleckner NW, McBain CJ (1990) The glycine coagonist site of the NMDA receptor. Adv Exp Med Biol 268:17–26
Dumitrescu ON, Protti DA, Majumdar S, Zeilhofer HU, Wässle H (2006) Ionotropic glutamate receptors of amacrine cells of the mouse retina. Vis Neurosci 23:79–90
Dutertre S, Becker CM, Betz H (2012) Inhibitory glycine receptors: an update. J Biol Chem 287:40216–40223
Eulenburg V, Becker K, Gomeza J, Schmitt B, Becker CM, Betz H (2006) Mutations within the human GLYT2 (SLC6A5) gene associated with hyperekplexia. Biochem Biophys Res Commun 348:400–405
Eulenburg V, Retiounskaia M, Papadopoulos T, Gomeza J, Betz H (2010) Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia Jul 58:1066–1073 (Epub 2010/05/15)
Farley FW, Soriano P, Steffen LS, Dymecki SM (2000) Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28:106–110
Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H (2003a) Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40:785–796
Gomeza J, Ohno K, Hulsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H (2003b) Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40:797–806
Haverkamp S, Wässle H (2000) Immunocytochemical analysis of the mouse retina. J Comp Neurol 424:1–23
Ishihara N, Armsen W, Papadopoulos T, Betz H, Eulenburg V (2010) Generation of a mouse line expressing Cre recombinase in glycinergic interneurons. Genesis 48:437–445
Kurolap A, Armbruster A, Hershkovitz T, Hauf K, Mory A, Paperna T, Hannappel E, Tal G, Nijem Y, Sella E et al (2016) Loss of glycine transporter 1 causes a subtype of glycine encephalopathy with arthrogryposis and mildly elevated cerebrospinal fluid glycine. Am J Hum Genet 99:1172–1180
Latal AT, Kremer T, Gomeza J, Eulenburg V, Hulsmann S (2010) Development of synaptic inhibition in glycine transporter 2 deficient mice. Mol Cell Neurosci 44:342–352 (Epub 2010/05/08)
Lee EJ, Kim HJ, Lim EJ, Kim IB, Kang WS, Oh SJ, Rickman DW, Chung JW, Chun MH (2004) AII amacrine cells in the mammalian retina show disabled-1 immunoreactivity. J Comp Neurol 470:372–381
Lee SC, Meyer A, Schubert T, Huser L, Dedek K, Haverkamp S (2015) Morphology and connectivity of the small bistratified A8 amacrine cell in the mouse retina. J Comp Neurol 523:1529–1547
Legendre P (2001) The glycinergic inhibitory synapse. Cell Mol Life Sci 58:760–793
Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermuller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K et al (2005) Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci 25:566–576
McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389:870–876
Nelson R, Famiglietti Jr EV, Kolb H (1978) Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol 41:472–483
Pfeiffer F, Simler R, Grenningloh G, Betz H (1984) Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA 81:7224–7227
Pourcho RG, Goebel DJ (1985) A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol 233:473–480
Pow DV (1998) Transport is the primary determinant of glycine content in retinal neurons. J Neurochem 70:2628–2636
Pow DV, Hendrickson AE (1999) Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci 16:231–239
Pow DV, Hendrickson AE (2000) Expression of glycine and the glycine transporter Glyt-1 in the developing rat retina. Vis Neurosci 17:1R–9R
Protti DA, Flores-Herr N, Li W, Massey SC, Wässle H (2005) Light signaling in scotopic conditions in the rabbit, mouse and rat retina: a physiological and anatomical study. J Neurophysiol 93:3479–3488 (Epub 2004/12/17)
Rees MI, Harvey K, Pearce BR, Chung SK, Duguid IC, Thomas P, Beatty S, Graham GE, Armstrong L, Shiang R et al (2006) Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat Genet 38:801–806
Regus-Leidig H, Ott C, Lohner M, Atorf J, Fuchs M, Sedmak T, Kremers J, Fejtova A, Gundelfinger ED, Brandstätter JH (2013) Identification and immunocytochemical characterization of Piccolino, a novel Piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS One 8:e70373
Rice DS, Curran T (2000) Disabled-1 is expressed in type AII amacrine cells in the mouse retina. J Comp Neurol 424:327–338
Rousseau F, Aubrey KR, Supplisson S (2008) The glycine transporter GlyT2 controls the dynamics of synaptic vesicle refilling in inhibitory spinal cord neurons. J Neurosci 28:9755–9768
Roux MJ, Supplisson S (2000) Neuronal and glial glycine transporters have different stoichiometries. Neuron 25:373–383
Roux MJ, Martinez-Maza R, Le Goff A, Lopez-Corcuera B, Aragon C, Supplisson S (2001) The glial and the neuronal glycine transporters differ in their reactivity to sulfhydryl reagents. J Biol Chem 276:17699–17705
Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B (1997) Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett 417:177–183
Schlosser L, Barthel F, Brandenburger T, Neumann E, Bauer I, Eulenburg V, Werdehausen R, Hermanns H (2015) Glycine transporter GlyT1, but not GlyT2, is expressed in rat dorsal root ganglion–possible implications for neuropathic pain. Neurosci Lett 600:213–219
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Supplisson S, Roux MJ (2002) Why glycine transporters have different stoichiometries. FEBS Lett 529:93–101
Vaney DI, Nelson JC, Pow DV (1998) Neurotransmitter coupling through gap junctions in the retina. J Neurosci 18:10594–10602
Wässle H (2004) Parallel processing in the mammalian retina. Nat Rev Neurosci 5:747–757
Yee BK, Balic E, Singer P et al (2006) Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci 26:3169–3181. https://doi.org/10.1523/JNEUROSCI.5120-05.2006
Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J (1995) Glycine transporters are differentially expressed among CNS cells. J Neurosci 15:3952–3969
Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM (2005) Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol 482:123–141
Acknowledgements
The authors thank Renate Kühnhauser, Marina Kleber and Freya Boggasch for excellent technical assistance. Parts of this work were supported by Grants from the Deutsche Forschungsgemeinschaft to V. E. (EU110/3-1 and EU110/6-1).
Author information
Authors and Affiliations
Contributions
VE, GK, and JHB designed experiments. GK, TS, SS, KH, JS, AJ, AF, and VE performed experiments. GK, TS, KH, JHB, AF, and VE analyzed data, VE wrote the manuscript with comments from all coauthors. All authors agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Eulenburg, V., Knop, G., Sedmak, T. et al. GlyT1 determines the glycinergic phenotype of amacrine cells in the mouse retina. Brain Struct Funct 223, 3251–3266 (2018). https://doi.org/10.1007/s00429-018-1684-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1684-3