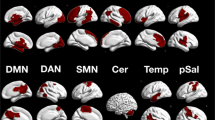
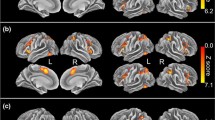
Abstract
Understanding functional connectivity of the amygdala with other brain regions, especially task modulated connectivity, is a critical step toward understanding the role of the amygdala in emotional processes and the interactions between emotion and cognition. The present study performed coordinate-based meta-analysis on studies of task modulated connectivity of the amygdala which used psychophysiological interaction (PPI) analysis. We first analyzed 49 PPI studies on different types of tasks using activation likelihood estimation (ALE) meta-analysis. Widespread cortical and subcortical regions showed consistent task modulated connectivity with the amygdala, including the medial frontal cortex, bilateral insula, anterior cingulate, fusiform gyrus, parahippocampal gyrus, thalamus, and basal ganglia. These regions were in general overlapped with those showed coactivations with the amygdala, suggesting that these regions and amygdala are not only activated together, but also show different levels of interactions during tasks. Further analyses with subsets of PPI studies revealed task specific functional connectivities with the amygdala that were modulated by fear processing, face processing, and emotion regulation. These results suggest a dynamic modulation of connectivity upon task demands, and provide new insights on the functions of the amygdala in different affective and cognitive processes. The meta-analytic approach on PPI studies may offer a framework toward systematical examinations of task modulated connectivity.



Similar content being viewed by others
References
Adolphs R, Tranel D, Damasio H, Damasio A (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672. doi:10.1038/372669a0
Adolphs R, Tranel D, Damasio AR (1998) The human amygdala in social judgment. Nature 393:470–474. doi:10.1038/30982
Adolphs R, Tranel D, Buchanan TW (2005) Amygdala damage impairs emotional memory for gist but not details of complex stimuli. Nat Neurosci 8:512–518. doi:10.1038/nn1413
Aggleton JP, Burton MJ, Passingham RE (1980) Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res 190:347–368. doi:10.1016/0006-8993(80)90279-6
Anderson AK, Phelps EA (2001) Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411:305–309. doi:10.1038/35077083
Baas D, Aleman A, Kahn RS (2004) Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev 45:96–103. doi:10.1016/j.brainresrev.2004.02.004
Bechara A (2000) Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10:295–307. doi:10.1093/cercor/10.3.295
Bechara A, Damasio H, Damasio AR (2003) Role of the amygdala in decision-making. Ann N Y Acad Sci 985:356–369. doi:10.1111/j.1749-6632.2003.tb07094.x
Blasi G, Lo Bianco L, Taurisano P, Gelao B, Romano R, Fazio L, Papazacharias A, Di Giorgio A, Caforio G, Rampino A, Masellis R, Papp A, Ursini G, Sinibaldi L, Popolizio T, Sadee W, Bertolino A (2009) Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci 29:14812–14819. doi:10.1523/JNEUROSCI.3609-09.2009
Bruneau EG, Jacoby N, Saxe R (2015) Empathic control through coordinated interaction of amygdala, theory of mind and extended pain matrix brain regions. Neuroimage 114:105–119. doi:10.1016/j.neuroimage.2015.04.034
Bullmore E, Sporns O (2012) The economy of brain network organization. Nat Rev Neurosci 13:336–349. doi:10.1038/nrn3214
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. doi:10.1016/j.cortex.2008.05.004
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94. doi:10.1006/nimg.2002.1136
Chan RCK, Di X, McAlonan GM, Gong Q (2011) Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull 37:177–188. doi:10.1093/schbul/sbp073
Cisler JM, Bush K, Steele JS (2013) A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage
Costafreda SG, Brammer MJ, David AS, Fu CHY (2008) Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58:57–70. doi:10.1016/j.brainresrev.2007.10.012
Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol M-J, van der Wee NJA, Veltman DJ, Roelofs K (2010) Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage 49:963–970. doi:10.1016/j.neuroimage.2009.08.023
Di X, Chan RCK, Gong Q (2009) White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 33:1390–1394. doi:10.1016/j.pnpbp.2009.08.020
Di X, Rypma B, Biswal BB (2014) Correspondence of executive function related functional and anatomical alterations in aging brain. Prog Neuropsychopharmacol Biol Psychiatry 48:41–50. doi:10.1016/j.pnpbp.2013.09.001
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. doi:10.1002/hbm.20718
Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59:2349–2361. doi:10.1016/j.neuroimage.2011.09.017
Ellison-Wright I, Bullmore E (2009) Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108:3–10. doi:10.1016/j.schres.2008.11.021
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008) The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 165:1015–1023. doi:10.1176/appi.ajp.2008.07101562
Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL (2005) BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp 25:185–198. doi:10.1002/hbm.20141
Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwartz N, Hussein AA, Smart LM, Sabatinelli D (2014) Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 45:202–211. doi:10.1016/j.neubiorev.2014.06.010
Friedel E, Schlagenhauf F, Sterzer P, Park SQ, Bermpohl F, Ströhle A, Stoy M, Puls I, Hägele C, Wrase J, Büchel C, Heinz A (2009) 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology 205:261–271. doi:10.1007/s00213-009-1536-1
Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997) Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229
Friston KJ, Harrison L, Penny W (2003) Dynamic causal modelling. Neuroimage 19:1273–1302. doi:10.1016/S1053-8119(03)00202-7
Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P (2009) Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432
Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003) Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage 19:200–207
Gorka AX, Knodt AR, Hariri AR (2015) Basal forebrain moderates the magnitude of task-dependent amygdala functional connectivity. Soc Cogn Affect Neurosci 10:501–507. doi:10.1093/scan/nsu080
Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R (2011) Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56:881–889. doi:10.1016/j.neuroimage.2011.02.064
Herrington JD, Taylor JM, Grupe DW, Curby KM, Schultz RT (2011) Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage 56:2348–2355. doi:10.1016/j.neuroimage.2011.03.072
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015) Large-scale network dysfunction in major depressive disorder. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2015.0071
Kim J, Horwitz B (2008) Investigating the neural basis for fMRI-based functional connectivity in a blocked design: application to interregional correlations and psycho-physiological interactions. Magn Reson Imaging 26:583–593. doi:10.1016/j.mri.2007.10.011
Koelsch S, Skouras S, Fritz T, Herrera P, Bonhage C, Küssner MB, Jacobs AM (2013) The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. Neuroimage 81:49–60. doi:10.1016/j.neuroimage.2013.05.008
Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014) Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage 87:345–355. doi:10.1016/j.neuroimage.2013.11.001
Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005a) ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25:155–164. doi:10.1002/hbm.20136
Laird AR, Lancaster JL, Fox PT (2005b) BrainMap: the social evolution of a human brain mapping database. Neuroinformatics 3:65–78
Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutiérrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL (2010) Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage 51:677–683. doi:10.1016/j.neuroimage.2010.02.048
Laird AR, Riedel MC, Sutherland MT, Eickhoff SB, Ray KL, Uecker AM, Fox PM, Turner JA, Fox PT (2015) Neural architecture underlying classification of face perception paradigms. Neuroimage 119:70–80. doi:10.1016/j.neuroimage.2015.06.044
Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007) Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 28:1194–1205. doi:10.1002/hbm.20345
Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F (2007) Emotion induction after direct intracerebral stimulations of human amygdala. Cereb Cortex 17:1307–1313. doi:10.1093/cercor/bhl041
Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ (2012) Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage 62:1575–1581. doi:10.1016/j.neuroimage.2012.05.044
Lemogne C, Gorwood P, Boni C, Pessiglione M, Lehéricy S, Fossati P (2011) Cognitive appraisal and life stress moderate the effects of the 5-HTTLPR polymorphism on amygdala reactivity. Hum Brain Mapp 32:1856–1867. doi:10.1002/hbm.21150
Leonard CM, Rolls ET, Wilson FAW, Baylis GC (1985) Neurons in the amygdala of the monkey with responses selective for faces. Behav Brain Res 15:159–176. doi:10.1016/0166-4328(85)90062-2
Lowe MJ, Mock BJ, Sorenson JA (1998) Functional connectivity in single and multislice echo planar imaging using resting-state fluctuations. Neuroimage 7:119–132. doi:10.1006/nimg.1997.0315
Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, Xu H-S, Fu X-M, Hu X, Zhang D-R (2010) Addiction related alteration in resting-state brain connectivity. Neuroimage 49:738–744. doi:10.1016/j.neuroimage.2009.08.037
Madsen MK, Mc Mahon B, Andersen SB, Siebner HR, Knudsen GM, Fisher PM (2016) Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Soc Cogn Affect Neurosci 11:140–149. doi:10.1093/scan/nsv098
McLaren DG, Ries ML, Xu G, Johnson SC (2012) A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61:1277–1286. doi:10.1016/j.neuroimage.2012.03.068
Mende-Siedlecki P, Verosky SC, Turk-Browne NB, Todorov A (2013) Robust selectivity for faces in the human amygdala in the absence of expressions. J Cogn Neurosci 25:2086–2106. doi:10.1162/jocn_a_00469
Morris JS, Ohman A, Dolan RJ (1998) Conscious and unconscious emotional learning in the human amygdala. Nature 393:467–470. doi:10.1038/30976
Mothersill O, Morris DW, Kelly S, Rose EJ, Fahey C, O’Brien C, Lyne R, Reilly R, Gill M, Corvin AP, Donohoe G (2014) Effects of MIR137 on fronto-amygdala functional connectivity. Neuroimage 90:189–195. doi:10.1016/j.neuroimage.2013.12.019
Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009) The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44:893–905. doi:10.1016/j.neuroimage.2008.09.036
Packard MG, Teather LA (1998) Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol Learn Mem 69:163–203. doi:10.1006/nlme.1997.3815
Passamonti L, Rowe JB, Ewbank M, Hampshire A, Keane J, Calder AJ (2008) Connectivity from the ventral anterior cingulate to the amygdala is modulated by appetitive motivation in response to facial signals of aggression. Neuroimage 43:562–570. doi:10.1016/j.neuroimage.2008.07.045
Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ (2009) Personality predicts the brain’s response to viewing appetizing foods: the neural basis of a risk factor for overeating. J Neurosci 29:43–51. doi:10.1523/JNEUROSCI.4966-08.2009
Pessoa L (2014) Understanding brain networks and brain organization. Phys Life Rev 11:400–435. doi:10.1016/j.plrev.2014.03.005
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348. doi:10.1006/nimg.2002.1087
Phelps EA (2006) Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 57:27–53. doi:10.1146/annurev.psych.56.091103.070234
Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS (1997) A specific neural substrate for perceiving facial expressions of disgust. Nature 389:495–498. doi:10.1038/39051
Puglia MH, Lillard TS, Morris JP, Connelly JJ (2015) Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci USA. 1422096112. doi:10.1073/pnas.1422096112
Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ-F (2009) A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci USA 106:19191–19196. doi:10.1073/pnas.0907425106
Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, de Quervain DJ-F, Papassotiropoulos A (2010) Aversive stimuli lead to differential amygdala activation and connectivity patterns depending on catechol-O-methyltransferase Val158Met genotype. Neuroimage 52:1712–1719. doi:10.1016/j.neuroimage.2010.05.054
Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT (2010) Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31:173–184. doi:10.1002/hbm.20854
Roiser JP, de Martino B, Tan GCY, Kumaran D, Seymour B, Wood NW, Dolan RJ (2009) A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci 29:5985–5991. doi:10.1523/JNEUROSCI.0407-09.2009
Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009) Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. doi:10.1016/j.neuroimage.2008.11.030
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. doi:10.1089/brain.2012.0080
Sarter M, Markowitsch HJ (1985) Involvement of the amygdala in learning and memory: a critical review, with emphasis on anatomical relations. Behav Neurosci 99:342–380. doi:10.1037/0735-7044.99.2.342
Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, Sammer G, Vaitl D (2002) The insula is not specifically involved in disgust processing: an fMRI study. NeuroReport 13:2023–2026. doi:10.1097/00001756-200211150-00006
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007
Sladky R, Hoflich A, Kublbock M, Kraus C, Baldinger P, Moser E, Lanzenberger R, Windischberger C (2015) Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cereb Cortex 25:895–903. doi:10.1093/cercor/bht279
Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I (2012) Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37:241–249. doi:10.1503/jpn.110069
Stamatakis EA, Marslen-Wilson WD, Tyler LK, Fletcher PC (2005) Cingulate control of fronto-temporal integration reflects linguistic demands: a three-way interaction in functional connectivity. Neuroimage 28:115–121. doi:10.1016/j.neuroimage.2005.06.012
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, New York
Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002) Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16:765–780. doi:10.1006/nimg.2002.1131
Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 33:1–13. doi:10.1002/hbm.21186
Turner JA, Laird AR (2012) The cognitive paradigm ontology: design and application. Neuroinformatics 10:57–66. doi:10.1007/s12021-011-9126-x
Vytal K, Hamann S (2010) Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cogn Neurosci 22:2864–2885. doi:10.1162/jocn.2009.21366
Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998) Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18:411–418. 9412517
Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E (2006) Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci 26:9264–9271. doi:10.1523/JNEUROSCI.1016-06.2006
Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL (2016) Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp. doi:10.1002/hbm.23129
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670. doi:10.1038/nmeth.1635
Yoder KJ, Porges EC, Decety J (2015) Amygdala subnuclei connectivity in response to violence reveals unique influences of individual differences in psychopathic traits in a nonforensic sample. Hum Brain Mapp 36:1417–1428. doi:10.1002/hbm.22712
Young MP, Scannell JW, Burns GAPC, Blakemore C (1994) Analysis of connectivity: neural systems in the cerebral cortex. Rev Neurosci 5:227–250. doi:10.1515/REVNEURO.1994.5.3.227
Acknowledgments
We thank Dr. Suril Gohel for his insightful comments on an earlier version of this manuscript. This research was supported by Grants from NIH R01AG032088, R01DA038895, and NSFC (National Natural Science Foundation of China) 31100747.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Studies that were included in the current meta-analysis
Appendix: Studies that were included in the current meta-analysis
1. Akitsuki Y, Decety J (2009) Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage 47:722–34.
2. Amting JM, Miller JE, Chow M, Mitchell DG V (2009) Getting mixed messages: the impact of conflicting social signals on the brain’s target emotional response. Neuroimage 47:1950–9.
3. Amting JM, Greening SG, Mitchell DG V (2010) Multiple mechanisms of consciousness: the neural correlates of emotional awareness. J Neurosci 30:10039–47.
4. Banks SJ, Eddy KT, Angstadt M, et al. (2007) Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–12.
5. Bruneau EG, Jacoby N, Saxe R (2015) Empathic control through coordinated interaction of amygdala, theory of mind and extended pain matrix brain regions. Neuroimage 114:105–119.
6. Cohen MX, Elger CE, Weber B (2008) Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage 39:1396–407.
7. Comte M, Schön D, Coull JT, et al. (2016) Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb Cortex 26: 144–155.
8. Decety J, Porges EC (2011) Imagining being the agent of actions that carry different moral consequences: an fMRI study. Neuropsychologia 49:2994–3001.
9. Erk S, Mikschl A, Stier S, et al. (2010) Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci 30:15726–34.
10. Fakra E, Salgado-Pineda P, Delaveau P, et al. (2008) Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res 100:191–205.
11. Foland LC, Altshuler LL, Bookheimer SY, et al. (2008) Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res 162:27–37.
12. Gianaros PJ, Onyewuenyi IC, Sheu LK, et al. (2012) Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp 33:1700–16.
13. Gold AL, Morey RA, McCarthy G (2015) Amygdala-prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol Psychiatry 77:394–403.
14. Grabenhorst F, Schulte FP, Maderwald S, Brand M (2013) Food labels promote healthy choices by a decision bias in the amygdala. Neuroimage 74:152–63.
15. Hermans EJ, Henckens MJAG, Roelofs K, Fernández G (2012) Fear bradycardia and activation of the human periaqueductal grey. Neuroimage 66C:278–287.
16. Herrmann MJ, Boehme S, Becker MPI, Tupak S V., Guhn A, Schmidt B, et al. (2016): Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum Brain Mapp 37:1091-1102.
17. Iidaka T, Omori M, Murata T, et al. (2001) Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J Cogn Neurosci 13:1035–47.
18. Kanske P, Heissler J, Schönfelder S, et al. (2011) How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 21:1379–88.
19. Kienast T, Hariri AR, Schlagenhauf F, et al. (2008) Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci 11:1381–2.
20. Koelsch S, Skouras S, Fritz T, et al. (2013) The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. Neuroimage 81:49–60.
21. Larson CL, Aronoff J, Sarinopoulos IC, Zhu DC (2009) Recognizing threat: a simple geometric shape activates neural circuitry for threat detection. J Cogn Neurosci 21:1523–35.
22. Maquet P, Phillips C (1998) Functional brain imaging of human sleep. J Sleep Res 7 Suppl 1:42–7.
23. Meier ML, Stämpfli P, Vrana A, Humphreys BK, Seifritz E, Hotz-Boendermaker S (2015): Fear avoidance beliefs in back pain-free subjects are reflected by amygdala-cingulate responses. Front Hum Neurosci. 9:424.
24. Mende-Siedlecki P, Verosky SC, Turk-Browne NB, Todorov A (2013) Robust selectivity for faces in the human amygdala in the absence of expressions. J Cogn Neurosci 25:2086–106.
25. Molapour T, Golkar A, Navarrete CD, Haaker J, Olsson A (2015) Neural correlates of biased social fear learning and interaction in an intergroup context. Neuroimage. 121:171–183.
26. Monk CS, Telzer EH, Mogg K, et al. (2008) Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 65:568–76.
27. Morris JS, Friston KJ, Büchel C, et al. (1998) A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121(Pt 1):47–57.
28. Morris JS, Dolan RJ (2001) Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci 21:5304–10.
29. Mukherjee P, Whalley HC, McKirdy JW, et al. (2014) Altered amygdala connectivity within the social brain in schizophrenia. Schizophr Bull 40:152–60.
30. Murray BD, Kensinger EA (2014) The route to an integrative associative memory is influenced by emotion. PLoS One 9:e82372.
31. Pasley BN, Mayes LC, Schultz RT (2004) Subcortical discrimination of unperceived objects during binocular rivalry. Neuron 42:163–72.
32. Pichon S, Rieger SW, Vuilleumier P (2012) Persistent affective biases in human amygdala response following implicit priming with negative emotion concepts. Neuroimage 62:1610–21.
33. Ponz A, Khatami R, Poryazova R, et al. (2010) Reduced amygdala activity during aversive conditioning in human narcolepsy. Ann Neurol 67:394–8.
34. Sato W, Kochiyama T, Uono S, Yoshikawa S, Toichi M (2016) Direction of amygdala–neocortex interaction during dynamic facial expression processing. Cereb Cortex. bhw036.
35. Schienle A, Ubel S, Schöngaßner F, et al. (2014) Disgust regulation via placebo: an fMRI study. Soc Cogn Affect Neurosci 9:985–990.
36. Schmitgen MM, Walter H, Drost S, Rückl S, Schnell K (2016): Stimulus-dependent amygdala involvement in affective theory of mind generation. Neuroimage. 129: 450–459.
37. Skelly LR, Decety J (2012) Passive and motivated perception of emotional faces: qualitative and quantitative changes in the face processing network. PLoS One 7:e40371.
38. Spielberg JM, Forbes EE, Ladouceur CD, et al. (2015) Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc Cogn Affect Neurosci 10:408–415.
39. Sripada C, Angstadt M, Kessler D, et al. (2014) Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage 89:110–21.
40. Stegmayer K, Usher J, Trost S, et al. (2015) Disturbed cortico-amygdalar functional connectivity as pathophysiological correlate of working memory deficits in bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci 265:303–311.
41. Sterpenich V, D’Argembeau A, Desseilles M, et al. (2006) The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci 26:7416–23.
42. Stevens JS, Jovanovic T, Fani N, et al. (2013) Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47:1469–78.
43. Tottenham N, Shapiro M, Telzer EH, Humphreys KL (2012) Amygdala response to mother. Dev Sci 15:307–19.
44. Troiani V, Price ET, Schultz RT (2014) Unseen fearful faces promote amygdala guidance of attention. Soc Cogn Affect Neurosci 9:133–40.
45. Van Wingen GA, Geuze E, Vermetten E, Fernández G (2011) Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry 16:664–71.
46. Williams LM, Das P, Liddell BJ, et al. (2006) Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci 26:9264–71.
47. Winecoff A, Labar KS, Madden DJ, et al. (2011) Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci 6:165–76.
48. Xu P, Gu R, Broster LS, et al. (2013) Neural basis of emotional decision making in trait anxiety. J Neurosci 33:18641–53.
49. Yoder KJ, Porges EC, Decety J (2015) Amygdala subnuclei connectivity in response to violence reveals unique influences of individual differences in psychopathic traits in a nonforensic sample. Hum Brain Mapp. 36: 1417–1428.
Rights and permissions
About this article
Cite this article
Di, X., Huang, J. & Biswal, B.B. Task modulated brain connectivity of the amygdala: a meta-analysis of psychophysiological interactions. Brain Struct Funct 222, 619–634 (2017). https://doi.org/10.1007/s00429-016-1239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1239-4