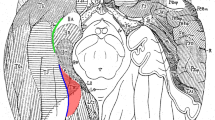
Abstract
The sulcal segments of the collateral sulcal complex on the medial part of the temporal lobe delineate the parahippocampal gyrus involved in memory processing from the laterally adjacent fusiform gyrus. The rhinal sulcus delineates the entorhinal cortex on the anterior portion of the parahippocampal gyrus. Posterior to the rhinal sulcus lies the collateral sulcus proper which delineates the parahippocampal cortex that occupies the posterior part of the parahippocampal gyrus. A small sulcus, the parahippocampal extension of the collateral sulcus, runs transversely within the parahippocampal gyrus. The rhinal sulcus, the collateral sulcus proper, and the parahippocampal extension of the collateral sulcus were identified on magnetic resonance images of 40 healthy human brains and probability maps were created to provide quantification of the location variability within standard stereotaxic space. These probability maps can act as a reference frame for the accurate identification of key components of the parahippocampal region and assist in the interpretation of structural and functional changes obtained in neuroimaging studies.








Similar content being viewed by others
References
Ad-Dab’bagh Y, Lyttelton O, Muehlboeck J-S, Lepage C, Einarson D, Mok K, Ivanov O, Vincent RD, Lerch J, Fombonne E, Evans AC (2006) The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. In: Corbetta M (ed) Proceedings of the 12th annual meeting of the organization for human brain mapping, Florence, Italy
Aguirre GK, D’Esposito M (1997) Environmental knowledge is subserved by separable dorsal/ventral neural areas. J Neurosci 17:2512–2518
Amaral DG, Insausti R, Cowan WM (1987) The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurology 264:326–355
Aminoff E, Gronau N, Bar M (2007) The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex 17:1493–1503
Amunts K et al (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol 210:343–352
Andrews TJ, Clarke A, Pell P, Hartley T (2010) Selectivity for low-level features of objects in the human ventral stream. NeuroImage 49:703–711
Arcaro MJ, McMains SA, Singer BD, Kastner S (2009) Retinotopic organization of human ventral visual cortex. J Neurosci 29:10638–10652
Augustinack JC, van der Kouwe AJ, Fischl B (2013) Medial temporal cortices in ex vivo magnetic resonance imaging. J Comp Neurol 521:4177–4188
Bachevalier J, Nemanic S (2008) Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus 18:64–80
Bar M, Aminoff E (2003) Cortical analysis of visual context Neuron 38:347–358
Bohbot VD, Corkin S (2007) Posterior parahippocampal place learning in H.M. Hippocampus 17:863–872
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig
Canto CB, Wouterlood FG, Witter MP (2008) What does the anatomical organization of the entorhinal cortex tell us? Neural Plast 2008:1–18
Chiavaras MM, LeGoualher G, Evans A, Petrides M (2001) Three-dimensional probabilistic atlas of the human orbitofrontal sulci in standardized stereotaxic space. NeuroImage 13:479–496
Collins DL, Neelin P, Peters TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205
Duvernoy H (1999) The human brain: surface, three-dimensional sectional anatomy and MRI, 2nd edn. Springer, Wien
Economo C, Koskinas GN (1925) Die Cytoarchitektur der Hirnrinde des erwachsenen Menschen. Springer, Wien
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335
Eickhoff SB, Heim S, Zilles K, Amunts K (2006) Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 32:570–582
Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 36:511–521
Epstein R, Kanwisher N (1998) A cortical representation of the local visual environment Nature 392:598–601
Epstein R, Harris A, Stanley D, Kanwisher N (1999) The parahippocampal place area: recognition, navigation, or encoding? Neuron 23:115–125
Epstein R, Graham KS, Downing PE (2003) Viewpoint-specific scene representations in human parahippocampal cortex. Neuron 37:865–876
Fischl B, Stevens AA, Rajendran N, Yeo BT, Greve DN, Van Leemput K, Polimeni JR, Kakunoori S, Buckner RL, Pacheco J, Salat DH, Melcher J, Frosch MP, Hyman BT, Grant PE, Rosen BR, van der Kouwe AJ, Wiggins GC, Wald LL, Augustinack JC (2009) Predicting the location of entorhinal cortex from MRI. NeuroImage 47:8–17
Germann J, Robbins S, Halsband U, Petrides M (2005) Precentral sulcal complex of the human brain: morphology and statistical probability maps. J Comp Neurol 493:334–356
Gloor P (1997) The temporal lobe and limbic system. Oxford University Press, New York
Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL (2006) Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 9:58–66
Hanke J (1997) Sulcal pattern of the anterior parahippocampal gyrus in the human adult. Ann Anat 179:335–339
Henderson JM, Larson CL, Zhu DC (2008) Full scenes produce more activation than close-up scenes and scene-diagnostic objects in parahippocampal and retrosplenial cortex: an fMRI study. Brain Cogn 66:40–49
Huntgeburth SC, Petrides M (2012) Morphological patterns of the collateral sulcus in the human brain. Eur J Neurosci 35:1295–1311
Iaria G, Petrides M (2007) Occipital sulci of the human brain: variability and probability maps. J Comp Neurol 501:243–259
Iaria G, Robbins S, Petrides M (2008) Three-dimensional probabilistic maps of the occipital sulci of the human brain in standardized stereotaxic space. Neuroscience 151:174–185
Insausti R (1993) Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus 3(S1):19–26
Insausti R, Amaral DG (2004) Hippocampal formation. In: Paxinos G, Mai JK (eds) The human nervous system, 2nd edn. Elsevier Academic Press, San Diego, pp 871–913
Insausti R, Amaral DG, Cowan WM (1987) The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol 264:356–395
Insausti R, Tunon T, Sobreviela T, Insausti AM, Gonzalo LM (1995) The human entorhinal cortex: a cytoarchitectonic analysis. J Comp Neurol 355:171–198
Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A (1998) MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol 19:659–671
Janzen G, van Turennout M (2004) Selective neural representation of objects relevant for navigation. Nat Neurosci 7:673–677
Kim H, Bernasconi N, Bernhardt B, Colliot O, Bernasconi A (2008) Basal temporal sulcal morphology in healthy controls and patients with temporal lobe epilepsy. Neurology 70:2159–2165
Köhler S, Crane J, Milner B (2002) Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus 12:718–723
Krimer LS, Hyde TM, Herman MM, Saunders RC (1997) The entorhinal cortex: an examination of cyto- and myeloarchitectonic organization in humans. Cereb Cortex 7:722–731
Litman L, Awipi T, Davachi L (2009) Category-specificity in the human medial temporal lobe cortex. Hippocampus 19:308–319
Malkova L, Mishkin M (2003) One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci 23:1956–1965
Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995a) A probabilistic atlas of the human brain: theory and rationale for its development the international consortium for brain mapping (ICBM). NeuroImage 2:89–101
Mazziotta JC, Toga AW, Evans AC, Fox PT, Lancaster JL (1995b) Digital brain atlases Trends in Neurosciences 18:210–211
Mazziotta J et al (2001) A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond, Ser B 356:1293–1322
Meunier M, Bachevalier J, Mishkin M, Murray EA (1993) Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci 13:5418–5432
Milner B (1968) Disorders of memory after brain lesion in man: preface: material-specific and generalized memory loss. Neuropsychologia 6:175–179
Milner B, Johnsrude I, Crane J (1997) Right medial temporal-lobe contribution to object-location memory. Philos Trans R Soc Lond Ser B Biol Sci 352:1469–1474
Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F (1997) Hierarchical organization of cognitive memory. Philos Trans R Soc Lond Ser B Biol Sci 352:1461–1467
Novak K, Czech T, Prayer D, Dietrich W, Serles W, Lehr S, Baumgartner C (2002) Individual variations in the sulcal anatomy of the basal temporal lobe and its relevance for epilepsy surgery: an anatomical study performed using magnetic resonance imaging. J Neurosurg 96:464–473
Ono M, Kubik S, Abernathey CD (1990) Atlas of the Cerebral Sulci. Thieme, Stuttgart
Paquette V, Levesque J, Mensour B, Leroux JM, Beaudoin G, Bourgouin P, Beauregard M (2003) “Change the mind and you change the brain”: effects of cognitive-behavioral therapy on the neural correlates of spider phobia. NeuroImage 18:401–409
Paus T et al (1996) In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol 376:664–673
Petrides M (2012) The human cerebral cortex: an MRI atlas of the sulci and gyri in MNI stereotaxic space. Academic Press, New York
Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ (2004) Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci 19:1939–1949
Reber PJ, Wong EC, Buxton RB (2002) Encoding activity in the medial temporal lobe examined with anatomically constrained fMRI analysis. Hippocampus 12:363–376
Retzius G (1896) Das Menschenhirn: Studien der makroskopischen. Morphologie Norstedt and Soener, Stockholm
Sato N, Nakamura K (2003) Visual response properties of neurons in the parahippocampal cortex of monkeys. J Neurophysiol 90:876–886
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Segal E, Petrides M (2012) The morphology and variability of the caudal rami of the superior temporal sulcus. Eur J Neurosci 36:2035–2053
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Smith EG (1904) Studies in the morphology of the human brain. Records of the Egyption Government School of Medicine Egyptians - No.1. Occipital Reg 2:124–173
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Squire LR, Zola-Morgan S (1988) Memory: brain systems and behavior. Trends Neurosci 11:170–175
Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380–1386
Staresina BP, Duncan KD, Davachi L (2011) Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci 31:8739–8747
Sulpizio V, Committeri G, Lambrey S, Berthoz A, Galati G (2013) Selective role of lingual/parahippocampal gyrus and retrosplenial complex in spatial memory across viewpoint changes relative to the environmental reference frame. Behav Brain Res 242:62–75
Suzuki WA, Amaral DG (1994) Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol 350:497–533
Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG (1993) Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci 13:2430–2451
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, New York
Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M (1999) Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci 11:3033–3046
Van Hoesen GW (1982) The parahippocampal gyrus: new observations regarding its cortical connections in the monkey. Trends Neurosci 5:345–350
Van Hoesen GW, Pandya DN (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res 95:1–24
Van Hoesen GW, Pandya DN, Butters N (1972) Cortical afferents to the entorhinal cortex of the Rhesus monkey. Science 175:1471–1473
Vogt BA, Vogt LJ, Perl DP, Hof PR (2001) Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J Comp Neurol 438:353–376
Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA (1989) Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci 9:4355–4370
Zola-Morgan S, Squire LR, Ramus SJ (1994) Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 4:483–495
Acknowledgments
We thank the National Institute of Biomedical Imaging and BioEngineering (principal investigator: John Mazziotta, MD, Ph.D.) for funding the International Consortium for Brain Mapping (ICBM) brains that were used in the present study. Furthermore, we thank Dr. Claude Lepage for technical help in image-processing necessary to register the entorhinal cortex probability map into the stereotaxic space used in the present study to allow comparison with the probability maps of the rhinal sulcus and the collateral sulcus proper. We also thank Dr. Veronika Zlatkina for helpful discussions and Trisanna Sprung-Much for help with manuscript revisions.
Funding
The research was supported by the Canadian Institutes of Health Research (CIHR) Grant MOP-130361 and the Foundation Grant FDN-143212 to M.P. Funding for the International Consortium for Brain Mapping (ICBM) brains was provided by the National Institute of Biomedical Imaging and BioEngineering (Principal Investigator: John Mazziotta, MD, Ph.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing financial or non-financial interests. All research was conducted in compliance with ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00429-016-1269-y.
Rights and permissions
About this article
Cite this article
Huntgeburth, S.C., Petrides, M. Three-dimensional probability maps of the rhinal and the collateral sulci in the human brain. Brain Struct Funct 221, 4235–4255 (2016). https://doi.org/10.1007/s00429-016-1189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1189-x