Abstract
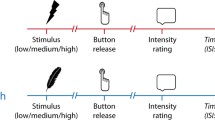
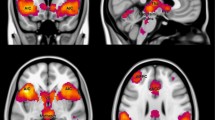
Behavioral inhibition has classically been considered to rely upon a neural network centered at the right inferior frontal cortex [rIFC; Aron et al. (8:170–177, 2004; 18:177–185, 2014)]. However, the vast majority of inhibition studies have entailed exogenous stop signals instructing participants to withhold responding. More recent work has begun to examine the neural underpinnings of endogenous inhibition, revealing a distinct cortical basis in the dorsal fronto-median cortex [dFMC; Brass and Haggard (27:9141–9145, 2007); Kühn et al. (30:2834–3843, 2009)]. Yet, contrary to everyday experiences of voluntary behavioral suppression, the paradigms employed to investigate action inhibition have thus far been somewhat artificial, and involve little persuasive motivation to act. Accordingly, the present fMRI study seeks to compare and contrast intentional with instructed inhibition in a novel pain paradigm that recruits ‘hot’ incentive response systems. Participants received increasing thermal stimulation to their inner wrists, and were required to occasionally withhold their natural impulse to withdraw from the compelling pain sensation at peak temperature, in both instructed and free-choice conditions. Consistent with previous research, we observed inhibition-related activity in the dFMC and the rIFC. However, these regions displayed equivalent activation levels for both inhibition types. These data extend previous research by demonstrating that under ecologically valid conditions with a strong motivation to act, both stopping networks operate in concert to enable suppression of unwanted behavior.






Similar content being viewed by others
Notes
We use the term ‘endogenous’ synonymously with ‘voluntary’ and ‘intentional’ to denote an internal locus of the decision to perform or withhold an action.
This temperature is henceforth referred to as the individual participant’s tolerance threshold.
A similar pattern of results was observed with a spherical ROI centered on the peak amPFC coordinate derived from the present study. However, these results are not displayed here due to the non-independence of ROI selection and analysis.
References
Aron AR, Poldrack RA (2006) Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci Off J Soc Neurosci 26:2424–2433
Aron AR, Robbins TW, Poldrack RA (2004) Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8(4):170–177
Aron AR, Robbins TW, Poldrack RA (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18(4):177–185
Baumeister RF, Vohs KD, Tice DM (2007) The strength model of self-control. Curr Dir Psychol Sci 16(6):351–355
Brass M, Haggard P (2007) To do or not to do: the neural signature of self-control. J Neurosci Off J Soc Neurosci 27(34):9141–9145
Brass M, Haggard P (2008) The what, when, whether model of intentional action. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 14:319–325
Brass M, Haggard P (2010) The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct Funct 214:603–610
Brass M, Derrfuss J, von Cramon DY (2005) The inhibition of imitative and overlearned repsonses: a functional double dissociation. Neuropsychologia 43:89–98
Brass M, Ruby P, Spengler S (2009) Inhibition of imitative behavior and social cognition. Philos Trans R Soc Biol Sci 364:2359–2367
Brett M, Anton JLL, Valabregue R, Poline JBB (2002). Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June 2–6, 2002. In: NeuroImage (Vol. 16, p. abstract 497)
Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Cohen MS (2007) Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry 62:642–651
Cai W, George JS, Verbruggen F, Chambers CD, Aron AR (2012) The role of the right presupplementary motor area in stopping action: two studies with event-related transcranial magnetic stimulation. J Neurophysiol 108(2):380–389
Campbell BA, Misanin JR (1969) Basic drives. Annu Rev Psychol 20:57–84
Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD (2008) Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry 63:293–300
Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y (2007) Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci 19:69–80
Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009) Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci Off J Soc Neurosci 29:15870–15877
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335
Elliot AJ (2006) The hierarchical model of approach-avoidance motivation. Motiv Emot 30(2):111–116
Filevich E, Kühn S, Haggard P (2012) Intentional inhibition in human action: the power of “no”. Neurosci Biobehav Rev 36(4):1107–1118
Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WPM, Ridderinkhof KR (2008) Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci Off J Soc Neurosci 28:9790–9796
Friston KJ, Frith CD, Frackowiak RS, Turner R (1995) Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 2(2):166–172
Garavan H, Ross TJ, Stein EA (1999) Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA 96:8301–8306
Garavan H, Ross TJ, Murphy K, Roche AP, Stein EA (2002) Dissociable executive functions in the dynamic control of behavior: inhibition, error detection and correction. NeuroImage 17:1820–1829
Garcia-Larrea L, Peyron R (2013) Pain matrices and neuropathic pain matrices: a review. Pain 154(Suppl 1):S29–S43 (Melzack 1990)
Haggard P (2008) Human volition: towards a neuroscience of will. Nat Rev Neurosci 9(12):934–946
Herz DM, Christensen MS, Bruggemann N, Hulme OJ, Ridderinkhof KR, Madsen KH, Siebner HR (2014) Motivational tuning of fronto-subthalamic connectivity facilitates control of action impulses. J Neurosci 34(9):3210–3217
Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, Kennard C (2007) The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain J Neurol 130:1525–1537
Ingvar M (1999) Pain and functional imaging. Philos Trans R Soc Lond 354(1387):1347–1358
Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, Matthews PM (2004) Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101(36):13335–13340
Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S (2006) Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci 1:56–64
Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI (2009) Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12(5):535–540
Kühn S, Haggard P, Brass M (2009) Intentional inhibition: how the “veto-area” exerts control. Hum Brain Mapp 30(9):2834–2843
Kühn S, Gallinat J, Brass M (2011) “Keep calm and carry on”: structural correlates of expressive suppression of emotions. PLoS One 6:e16569
Kühn S, Haggard P, Brass M (2014) Differences between endogenous and exogenous emotion inhibition in the human brain. Brain Struct Funct 219(3):1129–1138
Lynn MT, Muhle-Karbe PS, Brass M (2014) Controlling the self: the role of the dorsal frontomedian cortex in intentional inhibition. Neuropsychologia 65:247–254
Metcalfe J, Mischel W (1999) A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev 106(1):3–19
Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, Shoda Y (2011) “Willpower” over the life span: decomposing self-regulation. Soc Cogn Affect Neurosci 6(2):252–256
Mitchell JP, Banaji MR, Macrae CN (2005) The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci 17:1306–1315
Morsella E (2005) The function of phenomenal states: supramodular interaction theory. Psychological Review 112(4):1000–1021
Mueller V, Brass M, Waszak F, Prinz W (2007) The role of the preSMA and the rostral cingulate zone in internally selected actions. NeuroImage 37(4):1354–1361
Muraven M (2010) Practicing self-control lowers the risk of smoking lapse. Psychol Addict Behav 24(3):446–452
Muraven M, Baumeister RF (2000) Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol Bull 126(2):247–259
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Peyron R, Laurent B, García-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin Clin Neurophysiol 30(5):263–288
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science (N Y) 277:968–971
Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N (2012) Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 59(3):2860–2870
Tangney JP, Baumeister RF, Boone AL (2004) High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Personal 72(2):271–324
Towse JN, Neil D (1998) Analyzing human random generation behavior: A review of methods used and a computer program for describing performance. Behav Res Methods Instrum Comput 30(4):583–591
Verbruggen F, Logan GD (2008) Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12:418–424
Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Cohen JD (2004) Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (N Y) 303:1162–1167
Acknowledgments
We thank Pieter Vandemaele for technical support and Paul S. Muhle-Karbe for helpful comments on the manuscript. This work was supported by the European Science Foundation’s EUROVETO project (09-ECRP-020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lynn, M.T., Demanet, J., Krebs, R.M. et al. Voluntary inhibition of pain avoidance behavior: an fMRI study. Brain Struct Funct 221, 1309–1320 (2016). https://doi.org/10.1007/s00429-014-0972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0972-9