Abstract
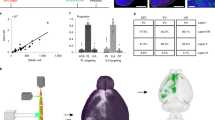
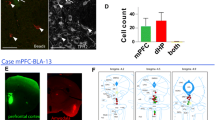
The median raphe region (MRR) is thought to be serotonergic and plays an important role in the regulation of many cognitive functions. In the hippocampus (HIPP), the MRR exerts a fast excitatory control, partially through glutamatergic transmission, on a subpopulation of GABAergic interneurons that are key regulators of local network activity. However, not all receptors of this connection in the HIPP and in synapses established by MRR in other brain areas are known. Using combined anterograde tracing and immunogold methods, we show that the GluN2A subunit of the NMDA receptor is present in the synapses established by MRR not only in the HIPP, but also in the medial septum (MS) and in the medial prefrontal cortex (mPFC) of the mouse. We estimated similar amounts of NMDA receptors in these synapses established by the MRR and in local adjacent excitatory synapses. Using retrograde tracing and confocal laser scanning microscopy, we found that the majority of the projecting cells of the mouse MRR contain the vesicular glutamate transporter type 3 (vGluT3). Furthermore, using double retrograde tracing, we found that single cells of the MRR can innervate the HIPP and mPFC or the MS and mPFC simultaneously, and these double-projecting cells are also predominantly vGluT3-positive. Our results indicate that the majority of the output of the MRR is glutamatergic and acts through NMDA receptor-containing synapses. This suggests that key forebrain areas receive precisely targeted excitatory input from the MRR, which is able to synchronously modify activity in those regions via individual MRR cells with dual projections.





Similar content being viewed by others
References
Acsády L, Halasy K, Freund TF (1993) Calretinin is present in non-pyramidal cells of the rat hippocampus–III. Their inputs from the median raphe and medial septal nuclei. Neuroscience 52(4):829–841
Acsády L, Arabadzisz D, Katona I, Freund TF (1996) Topographic distribution of dorsal and median raphe neurons with hippocampal, septal and dual projection. Acta Biol Hung 47(1–4):9–19
Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S (2010) vGluT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci 30(6):2198–2210. doi:10.1523/JNEUROSCI.5196-09.2010
Arens J, Stabel J, Heinemann U (1992) Pharmacological properties of excitatory amino acid induced changes in extracellular calcium concentration in rat hippocampal slices. Can J Physiol Pharmacol 70(Suppl):S194–S205
Assaf SY, Miller JJ (1978) The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience 3(6):539–550
Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179(3):641–667. doi:10.1002/cne.901790311
Aznar S, Qian ZX, Knudsen GM (2004) Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience 124(3):573–581. doi:10.1016/j.neuroscience.2003.12.020 (pii: S0306452203009333)
Bang SJ, Jensen P, Dymecki SM, Commons KG (2012) Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci 35(1):85–96. doi:10.1111/j.1460-9568.2011.07936.x
Chesnoy-Marchais D, Barthe JY (1996) Voltage-dependent block of NMDA responses by 5-HT agonists in ventral spinal cord neurones. Br J Pharmacol 117(1):133–141
de Bartolomeis A, Buonaguro EF, Iasevoli F (2013) Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology 225(1):1–19. doi:10.1007/s00213-012-2921-8
Dederen PJ, Gribnau AA, Curfs MH (1994) Retrograde neuronal tracing with cholera toxin B subunit: comparison of three different visualization methods. Histochem J 26 (11):856–862
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51(1):7–61
Freund TF, Antal M (1988) GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336(6195):170–173. doi:10.1038/336170a0
Freund TF, Gulyás AI, Acsády L, Görcs T, Tóth K (1990) Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci USA 87(21):8501–8505
Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M (2003) Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci USA 100(8):4855–4860. doi:10.1073/pnas.0830996100
Hamorsky KT, Kouokam JC, Bennett LJ, Baldauf KJ, Kajiura H, Fujiyama K, Matoba N (2013) Rapid and scalable plant-based production of a cholera toxin B subunit variant to aid in mass vaccination against cholera outbreaks. PLoS Negl Trop Dis 7(3):e2046
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27(6):555–579
Hensler JG (2006) Serotonergic modulation of the limbic system. Neurosci Biobehav Rev 30(2):203–214. doi:10.1016/j.neubiorev.2005.06.007 (pii: S0149-7634(05)00118-1)
Jackson J, Dickson CT, Bland BH (2008) Median raphe stimulation disrupts hippocampal theta via rapid inhibition and state-dependent phase reset of theta-related neural circuitry. J Neurophysiol 99(6):3009–3026. doi:10.1152/jn.00065.2008
Jackson J, Bland BH, Antle MC (2009) Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter vGluT3. Synapse 63(1):31–41. doi:10.1002/syn.20581
Klausberger T (2009) GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci 30(6):947–957. doi:10.1111/j.1460-9568.2009.06913.x
Köhler C, Steinbusch H (1982) Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience 7(4):951–975
Köhler C, Chan-Palay V, Steinbusch H (1982) The distribution and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and fluorescent retrograde tracing study in the rat. J Comp Neurol 209(1):91–111. doi:10.1002/cne.902090109
Kosofsky BE, Molliver ME (1987) The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal to median raphe nuclei. Synapse 1(2):153–168. doi:10.1002/syn.890010204
Lanciego JL, Wouterlood FG (2011) A half century of experimental neuroanatomical tracing. J Chem Neuroanat 42(3):157–183. doi:10.1016/j.jchemneu.2011.07.001
Leranth C, Vertes RP (1999) Median raphe serotonergic innervation of medial septum/diagonal band of broca (MSDB) parvalbumin-containing neurons: possible involvement of the MSDB in the desynchronization of the hippocampal EEG. J Comp Neurol 410(4):586–598. doi:10.1002/(SICI)1096-9861(19990809)410:4<586:AID-CNE6>3.0.CO;2-H
Liang X, Arvanov VL, Wang RY (1998) Inhibition of NMDA-receptor mediated response in the rat medial prefrontal cortical pyramidal cells by the 5-HT3 receptor agonist SR 57227A and 5-HT: intracellular studies. Synapse 29(3):257–268. doi:10.1002/(SICI)1098-2396(199807)29:3<257:AID-SYN8>3.0.CO;2-5
MacLean JN, Schmidt BJ (2001) Voltage-sensitivity of motoneuron NMDA receptor channels is modulated by serotonin in the neonatal rat spinal cord. J Neurophysiol 86(3):1131–1138
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44(1):5–21. doi:10.1016/j.neuron.2004.09.012
Maura G, Marcoli M, Pepicelli O, Rosu C, Viola C, Raiteri M (2000) Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in human neocortex slices: involvement of 5-HT(2C) and 5-HT(1A) receptors. Br J Pharmacol 130(8):1853–1858. doi:10.1038/sj.bjp.0703510
McKenna JT, Vertes RP (2001) Collateral projections from the median raphe nucleus to the medial septum and hippocampus. Brain Res Bull 54(6):619–630 (pii: S0361923001004658)
McMahon LL, Kauer JA (1997) Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol 78(5):2493–2502
Morales M, Bloom FE (1997) The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci 17(9):3157–3167
Papp EC, Hajos N, Acsády L, Freund TF (1999) Medial septal and median raphe innervation of vasoactive intestinal polypeptide-containing interneurons in the hippocampus. Neuroscience 90(2):369–382
Paxinos G, Franklin KBJ (2012) The mouse brain in stereotaxic coordinates, 4th edn. Academic Press, Waltham
Puig MV, Santana N, Celada P, Mengod G, Artigas F (2004) In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex 14(12):1365–1375. doi:10.1093/cercor/bhh097
Ropert N, Guy N (1991) Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol 441:121–136
San Paulo A, García R (2000) High-resolution imaging of antibodies by tapping-mode atomic force microscopy: attractive and repulsive tip-sample interaction regimes. Biophys J 78(3):1599–1605. doi:10.1016/S0006-3495(00)76712-9
Semba K (2000) Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res 115(2):117–141
Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P (2004) GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (vGluT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci 19(3):552–569
Szabadits E, Cserép C, Szonyi A, Fukazawa Y, Shigemoto R, Watanabe M, Itohara S, Freund TF, Nyiri G (2011) NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J Neurosci 31(16):5893–5904. doi:10.1523/JNEUROSCI.5938-10.2011 (pii: 31/16/5893)
Takács VT, Freund TF, Gulyás AI (2008) Types and synaptic connections of hippocampal inhibitory neurons reciprocally connected with the medial septum. Eur J Neurosci 28(1):148–164. doi:10.1111/j.1460-9568.2008.06319.x
Takács VT, Freund TF, Nyiri G (2013) Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain. PLoS One 8(9):e72450. doi:10.1371/journal.pone.0072450
Varga C, Sík A, Lavallée P, Deschênes M (2002) Dendroarchitecture of relay cells in thalamic barreloids: a substrate for cross-whisker modulation. J Neurosci 22 (14):6186–6194
Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF (2009) Fast synaptic subcortical control of hippocampal circuits. Science 326(5951):449–453. doi:10.1126/science.1178307 (pii: 326/5951/449)
Varoqueaux F, Jamain S, Brose N (2004) Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol 83(9):449–456. doi:10.1078/0171-9335-00410
Vassilev PM, Mitchel J, Vassilev M, Kanazirska M, Brown EM (1997) Assessment of frequency-dependent alterations in the level of extracellular Ca2+ in the synaptic cleft. Biophys J 72(5):2103–2116. doi:10.1016/S0006-3495(97)78853-2
Vertes RP, Kocsis B (1997) Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81(4):893–926
Vertes RP, Martin GF (1988) Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol 275(4):511–541. doi:10.1002/cne.902750404
Vertes RP, Fortin WJ, Crane AM (1999) Projections of the median raphe nucleus in the rat. J Comp Neurol 407(4):555–582. doi:10.1002/(SICI)1096-9861(19990517)407:4<555:AID-CNE7>3.0.CO;2-E
Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y (1998) Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci 10(2):478–487
Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z (2005) Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci 25(23):5488–5501. doi:10.1523/JNEUROSCI.1187-05.2005
Zhang L, Ren G (2012) IPET and FETR: experimental approach for studying molecular structure dynamics by cryo-electron tomography of a single-molecule structure. PLoS One 7(1):e30249. doi:10.1371/journal.pone.0030249
Acknowledgments
We thank Emőke Szépné Simon, Katalin Lengyel, Katalin Iványi and Győző Goda for the excellent technical assistance and Dr. Ferenc Mátyás for technical discussions. We thank Dr. Viktor Varga for his comments on the previous version of the manuscript. The authors wish to thank László Barna, the Nikon Microscopy Center at IEM, Nikon Austria GmbH and Auro-Science Consulting Ltd for kindly providing technical support for fluorescent microscopy. This work was supported by the National Institutes of Health (grant number NS030549), National Office for Research and Technology–Hungarian Scientific Research Fund (NKTH-OTKA, grant number CNK77793, K83251) and European Research Council (grant number ERC-2011-ADG-294313, SERRACO). András Szőnyi was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/1-11-1-2012-0001 “National Excellence Program”. Gabor Nyiri was supported by a János Bolyai Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szőnyi, A., Mayer, M.I., Cserép, C. et al. The ascending median raphe projections are mainly glutamatergic in the mouse forebrain. Brain Struct Funct 221, 735–751 (2016). https://doi.org/10.1007/s00429-014-0935-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0935-1