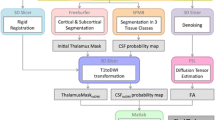
Abstract
To assess stable anatomical features of the human thalamus, an unbiased diffusion tensor parcellation approach was used to segment thalamic substructures with similar spatial orientation. We determined localization, size and individual variations of 21 thalamic clusters in a group of 63 healthy human subjects (32 males/31 females). The laterality differences accounted for ±6 % and gender differences for ±4 % of the thalamic volume. Consecutively, five stable clusters in the anterior, medial, lateral and posterior thalamus were selected, which were common to 90 % of all subjects and contained at least 10 voxels. These clusters could be assigned to the anteroventral nucleus (AN) group, the mediodorsal (MD) nucleus, the medial pulvinar (PuM), and the lateral nuclei group. The subcortical and cortical connectivity of these clusters revealed that: (1) the oblique cranio-caudal-oriented fibers of the AN cluster mainly connect to limbic structures, (2) the numerous dorso-frontal-oriented fibers of MD mainly project to the prefrontal cortex and the medial temporal lobe, (3) the fibers of the PuM running in parallel with the x-axis project to medio-occipital and medio-temporal areas and connect visual areas with the hippocampus and amygdala and via intrathalamic pathways with medio-frontal areas, and (4) the oblique caudo-cranial fibers of the two lateral clusters located anteriorly in the motor and posteriorly in the sensory thalamus are routing sensory–motor information from the brain stem via the internal capsule to pre- and peri-central regions of the cortex.







Similar content being viewed by others
Abbreviations
- AC:
-
Anterior commissure
- Acc:
-
Nucleus accumbens
- AD:
-
Anterodorsal nucleus
- AM:
-
Anteromedial nucleus
- Amy:
-
Amygdala
- AN:
-
Anterior cluster
- AV:
-
Anteroventral group
- BS:
-
Brainstem
- Cau:
-
Caudate nucleus
- CoG:
-
Center of gravity
- DDO:
-
Dominant diffusion orientations
- Den:
-
Dentate nucleus
- DTI:
-
Diffusion tensor imaging
- EPI:
-
Echo planar imaging
- Hip:
-
Hippocampus
- LA:
-
Lateral-anterior cluster
- LD:
-
Lateral dorsal group
- LP:
-
Lateral-posterior cluster
- MD:
-
Mediodorsal nucleus
- MED:
-
Medial cluster
- MRI:
-
Magnetic resonance imaging
- Pal:
-
Pallidum
- PC:
-
Posterior commissure
- PO:
-
Posterior cluster
- PuM:
-
Medial pulvinar
- Put:
-
Putamen
- Red:
-
Red nucleus
- SD:
-
Standard deviation
- T :
-
Tesla
- TE:
-
Echo time
- TR:
-
Repetition time
- VA:
-
Ventral anterior
- VLa:
-
Ventral lateral anterior
- VLp:
-
Ventral lateral posterior
- VM:
-
Ventral medial
- VP:
-
Ventral posterior complex
- VPI:
-
Ventral posterior inferior nucleus
- VPL:
-
Ventral posterolateral
- VPM:
-
Ventral posteromedial
References
Abosch A, Yacoub E, Ugurbil K, Harel N (2010) An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 Tesla. Neurosurgery 67:1745–1756
Aggleton JP, Mishkin M (1984) Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol 222:56–68
Akert K (1964) Comparative anatomy of frontal cortex and thalamo-frontal connections. In: Warren JM, Akert K (eds) The frontal granular cortex and behavior. McGraw-Hill, NewYork, pp 372–396
Alexander DC, Pierpaoli C, Basser PJ, Gee JC (2001) Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging 20:1131–1139
Amunts VV (2008) Individual variability in the structural asymmetry of the dorsomedial nucleus of the thalamus in men and women. Neurosci Behav Physiol 38:715–720
Baas D, Aleman A, Kahn RS (2004) Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev 45:96–103
Baron-Cohen S, Wheelwright S (2004) The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord 34:163–175
Behrens TEJ, Johansen-Berg H, Woolrich MW et al (2003a) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757
Behrens TEJ, Woolrich MW, Jenkinson M et al (2003b) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50:1077–1088
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132
Cook P, Bai Y, Gilani N et al. (2006) Camino: open-source diffusion-MRI reconstruction and processing. In: 14th scientific meeting of the international society for magnetic resonance in medicine, vol. 2759
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194
De Sousa TB, de Santana MAD, Ade MS et al (2013) Mediodorsal thalamic nucleus receives a direct retinal input in marmoset monkey (Callithrix jacchus): a subunit B cholera toxin study. Ann Anat 195:32–38
Deoni SCL, Rutt BK, Parrent AG, Peters TM (2007) Segmentation of thalamic nuclei using a modified k-means clustering algorithm and high-resolution quantitative magnetic resonance imaging at 1.5 T. NeuroImage 34:117–126
Fischer J, Whitney D (2012) Attention gates visual coding in the human pulvinar. Nat Commun 3:1051
Friston KJ, Jezzard P, Turner R (1994) Analysis of functional MRI time-series. Hum Brain Mapp 1:153–171
Fuster JM (1997) The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Lippincott-Raven, Philadelphia
Goebel R, Esposito F, Formisano E (2006) Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 27:392–401
Granziera C, Hadjikhani N, Arzy S et al (2011) In-vivo magnetic resonance imaging of the structural core of the Papez circuit in humans. Neuroreport 22:227–231
Gringel T, Schulz-Schaeffer W, Elolf E et al (2009) Optimized high-resolution mapping of magnetization transfer (MT) at 3 Tesla for direct visualization of substructures of the human thalamus in clinically feasible measurement time. J Magn Reson Imaging 29:1285–1292
Hebb AO, Ojemann GA (2013) The thalamus and language revisited. Brain Lang 126:99–108
Johansen-Berg H, Behrens TEJ, Sillery E et al (2005) Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex 15:31–39
Jones EG (2007) The Thalamus 2 Volume Set. Cambridge University Press
Jones DK, Cercignani M (2010) Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23:803–820
Krauth A, Blanc R, Poveda A et al (2010) A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 49:2053–2062
Kumar K, Mang S, Grodd W (2010) Consistency of automatic thalamus segmentation using DTI. Poster presented at the 16th annual meeting of the organization for human brain mapping, Barcelona (see supplement material)
Kumar K, Mang S, Reiterer S., Grodd W. (2011) DTI of the human thalamus: hemispheric and gender variability. Oral presentation in 17th annual meeting of the organization for human brain mapping, Québec (see supplement material)
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349
Lemaire J–J, Coste J, Ouchchane L et al (2007) Brain mapping in stereotactic surgery: a brief overview from the probabilistic targeting to the patient-based anatomic mapping. Neuroimage 37(Suppl 1):S109–S115
Lemaire J–J, Sakka L, Ouchchane L et al (2010) Anatomy of the human thalamus based on spontaneous contrast and microscopic voxels in high-field magnetic resonance imaging. Neurosurgery 66:161–172
Mai JK, Forutan F (2012) Thalamus. In: Mai Juergen K, Paxinos George (eds) Human nervous system, 3rd edn. Academic Press, San Diego, pp 618–677
Mai JK, Paxinos G, Voss T (2008) Atlas of the human brain. Academic Press, San Diego
Mang SC, Busza A, Reiterer S et al (2012) Thalamus segmentation based on the local diffusion direction: a group study. Magn Reson Med 67:118–126
Menzler K, Belke M, Wehrmann E et al (2011) Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 54:2557–2562
Morel A (2007) Stereotactic atlas of the human thalamus and basal ganglia. Informa Healthcare, New York, London
Niemann K, Mennicken VR, Jeanmonod D, Morel A (2000) The morel stereotactic atlas of the human thalamus: atlas-to-mr registration of internally consistent canonical model. NeuroImage 12:601–616
Nieuwenhuys R, Voogd J, Huijzen CV et al (2008) The human central nervous system, 4th edn. Springer-Verlag, Berlin Heidelberg, New York
Padmala S, Lim S-L, Pessoa L (2010) Pulvinar and affective significance: responses track moment-to-moment stimulus visibility. Front Hum Neurosci 4:64
Papez JW (1937) A proposed mechanism of emotion. Arch Neural Psychiat 38:725–743
Percheron G, François C, Talbi B et al (1996) The primate motor thalamus. Brain Res Brain Res Rev 22:93–181
Ray JP, Price JL (1992) The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain–prefrontal cortex topography. J Comp Neurol 323:167–197
Rohde GK, Barnett AS, Basser PJ et al (2004) Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med 51:103–114
Saalmann YB, Pinsk MA, Wang L et al (2012) The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337:753–756
Schultz T (2011) Segmenting thalamic nuclei: what can we gain from HARDI? In: Fichtinger G, Martel A, Peters T (eds) Medical image computing and computer-assisted intervention–MICCAI 2011. Springer, Berlin, Heidelberg, pp 141–148
Sherman SM, Guillery RW (2009) Exploring the thalamus and its role in cortical function. Mit Press, Cambridge
Shipp S (2003) The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci 358:1605–1624
Sladky R, Höflich A, Atanelov J et al (2012) Increased neural habituation in the amygdala and orbitofrontal cortex in social anxiety disorder revealed by fMRI. PLoS One 7:e50050
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Spinks R, Magnotta VA, Andreasen NC et al (2002) Manual and automated measurement of the whole thalamus and mediodorsal nucleus using magnetic resonance imaging. Neuroimage 17:631–642
Talairach J, David M, Tournoux P, Corredor H, Kvasina T (1957) Atlas d’Anatomie Ste′re′otaxique. Repe′rage Radiologique Indirect des Noyaux Gris Centraux, des Re′gions Me′sence′phalo-sous-Optique et Hypothalamique de l’Homme. Masson & Cie, Paris
Troiani V, Schultz RT (2013) Amygdala, pulvinar, and inferior parietal cortex contribute to early processing of faces without awareness. Front Hum Neurosci 7:241
Unrath A, Klose U, Grodd W et al (2008) Directional colour encoding of the human thalamus by diffusion tensor imaging. Neurosci Lett 434:322–327
Vrtička P, Sander D, Vuilleumier P (2013) Lateralized interactive social content and valence processing within the human amygdala. Front Hum Neurosci 6:358
Wager TD, Phan KL, Liberzon I, Taylor SF (2003) Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage 19:513–531
Wiegell MR, Tuch DS, Larsson HB, Wedeen VJ (2003) Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. NeuroImage 19:391–401
Acknowledgments
The digital model of the 3D anatomy of the thalamus according to the atlas of Morel (Krauth et al. 2010) was obtained by a written consent with Prof. G. Székely from the Computer Vision Laboratory of the ETH Zürich. We thank Susanne Reiterer for providing the data, Klaus Scheffler for giving access to analysis facilities, Bernd Kardatzki for technical support, Ute Habel, and Eugene Datta for reviewing the manuscript. The German research council (DFG) Grant GR 833/9-1 in part supported this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 1483 kb)
Supplementary material 3 (MP4 1180 kb)
Supplementary material 5 (MP4 1603 kb)
Rights and permissions
About this article
Cite this article
Kumar, V., Mang, S. & Grodd, W. Direct diffusion-based parcellation of the human thalamus. Brain Struct Funct 220, 1619–1635 (2015). https://doi.org/10.1007/s00429-014-0748-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0748-2