Abstract
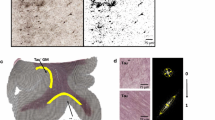
Diffusion tensor imaging (DTI) has become a valuable tool to investigate white matter integrity in the brain. DTI also gives contrast in gray matter, which has been relatively little explored in studies assessing post-injury structural abnormalities. The present study was designed to compare white and gray matter reorganization in the rat hippocampus after two epileptogenic brain injuries, status epilepticus (SE) and traumatic brain injury (TBI), using ex vivo high-resolution DTI. Imaging was performed at 6–12 months post-injury and findings were compared to histological analyses of Nissl, myelin, and Timm-stained preparations from the same animals. In agreement with the severity of histological damage, fractional anisotropy (FA), axial (D ||) and radial (D ⊥) diffusivities, and mean diffusivity (MD) measurements were altered in the order SE > TBI ipsilaterally > TBI contralaterally. After SE, the most severe abnormalities were found in the dentate gyrus and CA3b–c subfields, in which the mean FA was increased to 125 % (p < 0.001) and 143 % (p < 0.001) of that in controls, respectively. In both subfields, the change in FA was associated with an increase in D || (p < 0.01). In the stratum radiatum of the CA1, FA was decreased to 81 % of that in controls (p < 0.05) which was associated with an increase in D ⊥ (p < 0.01). After TBI, DTI did not reveal any major abnormalities in the dentate gyrus. In the ipsilateral CA3b–c, however, FA was increased to 126 % of that in controls (p < 0.01) and associated with a mild decrease in D ⊥ (p < 0.05). In the stratum radiatum of the ipsilateral CA1, FA was decreased to 88 % of that in controls (p < 0.05). Our data demonstrate that DTI reveals subfield-specific abnormalities in the hippocampus with remarkable qualitative and quantitative differences between the two epileptogenic etiologies, suggesting that DTI could be a valuable tool for follow-up of focal circuitry reorganization during the post-injury aftermath.










Similar content being viewed by others
Abbreviations
- D || :
-
Axial diffusivity
- D ⊥ :
-
Radial diffusivity
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- FPI:
-
Fluid percussion injury
- MD:
-
Mean diffusivity
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- SE:
-
Status epilepticus
- TBI:
-
Traumatic brain injury
References
Amaral DG, Insausti R (1990) Hippocampal formation. In: Paxinos G (ed) The human nervous system. Academic Press, San Diego, pp 711–755
Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31:571–591
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Basser PJ, Pierpaoli C (1998) A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med 39:928–934
Baulac M, Pitkänen A (2008) Research priorities in epilepsy for the next decade—a representative view of the European scientific community. Epilepsia
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455
Bennett RE, Mac Donald CL, Brody DL (2012) Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci Lett 513:160–165
Buckmaster PS (2012) Mossy fiber sprouting in the dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. Michael A Rogawski, Antonio V Delgado-Escueta, Jeffrey L Noebels, Massimo Avoli and Richard W Olsen, Bethesda
Buckmaster PS, Ingram EA, Wen X (2009) Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci 29(25):8259–8269
Budde MD, Janes L, Gold E, Turtzo LC, Frank JA (2011) The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 134:2248–2260
Cavazos JE, Golarai G, Sutula TP (1991) Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci 11:2795–2803
Chan KC, Cheng JS, Fan S, Zhou IY, Yang J, Wu EX (2012) In vivo evaluation of retinal and callosal projections in early postnatal development and plasticity using manganese-enhanced MRI and diffusion tensor imaging. NeuroImage 59:2274–2283
Christidi F, Bigler ED, McCauley SR, Schnelle KP, Merkley TL, Mors MB et al (2011) Diffusion tensor imaging of the perforant pathway zone and its relation to memory function in patients with severe traumatic brain injury. J Neurotrauma 28:711–725
Concha L, Gross DW, Wheatley BM, Beaulieu C (2006) Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage 32:1090–1099
Cross DJ, Cavazos JE (2007) Synaptic reorganization in subiculum and CA3 after early-life status epilepticus in the kainic acid rat model. Epilepsy Res 73:156–165
Dauguet J, Peled S, Berezovskii V, Delzescaux T, Warfield SK, Born R et al (2007) Comparison of fiber tracts derived from in vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. NeuroImage 37(2):530–538
de Carvalho Rangel C, Hygino Cruz LC Jr, Takayassu TC, Gasparetto EL, Domingues RC (2011) Diffusion MR imaging in central nervous system. Magn Reson Imaging Clin N Am 19:23–53
de Lanerolle NC, Lee TS, Spencer DD (2012) Histopathology of human epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. Michael A Rogawski, Antonio V Delgado-Escueta, Jeffrey L Noebels, Massimo Avoli and Richard W Olsen, Bethesda
Dityatev A, Fellin T (2008) Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol 4:235–247
Galanopoulou AS, Buckmaster PS, Staley KJ, Moshe SL, Perucca E, Engel J Jr et al (2012) Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia 53:571–582
Hauser WA (1997) Incidence and prevalence. In: Engel J Jr, Pedley TA (eds) Epilepsy: a comprehensive textbook. Lippincott-Raven Publishers, Philadelphia, pp 47–57
Hayward NM, Tuunanen PI, Immonen R, Ndode-Ekane XE, Pitkänen A, Gröhn O (2011) Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J Cereb Blood Flow Metab 31:166–177
Heidemann RM, Anwander A, Feiweier T, Knosche TR, Turner R (2012) k-space and q-space: combining ultra-high spatial and angular resolution in diffusion imaging using ZOOPPA at 7 T. NeuroImage 60:967–978
Horsfield MA, Jones DK (2002) Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases—a review. NMR Biomed 15:570–577
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841
Jones DK (2004) The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med 51(4):807–815
Kaufman et al (2005) Anatomical analysis of an aye–aye brain (Daubentonia madagascariensis, primates: Prosimii) combining histology, structural magnetic resonance imaging, and diffusion-tensor imaging. Anat Rec 287A:1026–1037
Kelley MS, Jacobs MP, Lowenstein DH, NINDS Epilepsy Benchmark Stewards (2009) The NINDS epilepsy research benchmarks. Epilepsia 50(3):579–582
Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A (2006) A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140:685–697
Kharatishvili I, Immonen R, Gröhn O, Pitkänen A (2007) Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain 130:3155–3168
Kuo LW, Lee CY, Chen JH, Wedeen VJ, Chen CC, Liou HH et al (2008) Mossy fiber sprouting in pilocarpine-induced status epilepticus rat hippocampus: a correlative study of diffusion spectrum imaging and histology. NeuroImage 41:789–800
Laitinen T, Sierra A, Pitkänen A, Gröhn O (2010) Diffusion tensor MRI of axonal plasticity in the rat hippocampus. NeuroImage 51:521–530
Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S (2007) Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. NeuroImage 36(4):1123–1138
Laurberg S, Zimmer J (1981) Lesion-induced sprouting of hippocampal mossy fiber collaterals to the fascia dentata in developing and adult rats. J Comp Neurol 200:433–459
Leergaard TB, White NS, de Crespigny A, Bolstad I, D’Arceuil H, Bjaalie JG et al (2010) Quantitative histological validation of diffusion MRI fiber orientation distributions in the rat brain. PLoS ONE 5(1):e8595
McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H et al (1989) Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28:233–244
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Nairismagi J, Pitkänen A, Narkilahti S, Huttunen J, Kauppinen RA, Gröhn OH (2006) Manganese-enhanced magnetic resonance imaging of mossy fiber plasticity in vivo. NeuroImage 30:130–135
Ndode-Ekane XE, Hayward N, Gröhn O, Pitkänen A (2010) Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience 166:312–332
Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J (2006) Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol 27(8):1776–1781
Nissinen J, Lukasiuk K, Pitkänen A (2001) Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus 11:299–310
Oechslin MS, Imfeld A, Loenneker T, Meyer M, Jancke L (2010) The plasticity of the superior longitudinal fasciculus as a function of musical expertise: a diffusion tensor imaging study. Front Hum Neurosci 3:76
Pajevic S, Pierpaoli C (1999) Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 42:526–540
Parekh MB, Carney PR, Sepulveda H, Norman W, King M, Mareci TH (2010) Early MR diffusion and relaxation changes in the parahippocampal gyrus precede the onset of spontaneous seizures in an animal model of chronic limbic epilepsy. Exp Neurol 224:258–270
Perez Y, Morin F, Beaulieu C, Lacaille JC (1996) Axonal sprouting of CA1 pyramidal cells in hyperexcitable hippocampal slices of kainate-treated rats. Eur J Neurosci 8:736–748
Pitkänen A (2010) Therapeutic approaches to epileptogenesis—hope on the horizon. Epilepsia 51(Suppl 3):2–17
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003) Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Res Med 49:177–182
Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P et al (2007) Angiogenesis is associated with blood–brain barrier permeability in temporal lobe epilepsy. Brain 130:1942–1956
Savander V, Go CG, Ledoux JE, Pitkänen A (1996) Intrinsic connections of the rat amygdaloid complex: projections originating in the accessory basal nucleus. J Comp Neurol 374:291–313
Shepherd TM, Ozarslan E, King MA, Mareci TH, Blackband SJ (2006) Structural insights from high-resolution diffusion tensor imaging and tractography of the isolated rat hippocampus. NeuroImage 32:1499–1509
Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P et al (2008) Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131:559–572
Sierra A, Laitinen T, Lehtimäki K, Rieppo L, Pitkänen A, Gröhn O (2011) Diffusion tensor MRI with tract-based spatial statistics and histology reveals undiscovered lesioned areas in kainate model of epilepsy in rat. Brain Struct Funct 216:123–135
Sigal T, Shmuel M, Mark D, Gil H, Anat A (2012) Diffusion tensor imaging of corpus callosum integrity in multiple sclerosis: correlation with disease variables. J Neuroimaging 22:33–37
Sloviter RS (1982) A simplified Timm stain procedure compatible with formaldehyde fixation and routine paraffin embedding of rat brain. Brain Res Bull 8:771–774
Sun SW, Neil JJ, Song SK (2003) Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med 50:743–748
Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS (2010) Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol 518:647–667
Wong M (2005) Modulation of dendritic spines in epilepsy: cellular mechanisms and functional implications. Epilepsy Behav 7:569–577
Yassa MA, Muftuler LT, Stark CE (2010) Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci USA 107:12687–12691
Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET et al (2011) Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry 69:80–89
Zhang J, van Zijl PC, Mori S (2002) Three-dimensional diffusion tensor magnetic resonance microimaging of adult mouse brain and hippocampus. NeuroImage 15:892–901
Zhang J, van Zijl PC, Laterra J, Salhotra A, Lal B, Mori S et al (2007) Unique patterns of diffusion directionality in rat brain tumors revealed by high-resolution diffusion tensor MRI. Magn Reson Med 58:454–462
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132(4):645–660
Acknowledgments
This study was supported by the Academy of Finland (A.S., O.G., A.P.), Sigrid Juselius Foundation (A.P.), and UEF-Brain strategic funding from the University of Eastern Finland. We thank Ms. Maarit Pulkkinen for assistance in histology, Dr. Jari Nissinen and Dr. Tamuna Bolkvadze for technical assistance, MSc Juha-Pekka Niskanen for drawing the diffusion ellipsoids, and Dr. Nick Hayward for revising the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Video 1 (MPG 1415 kb)
Supplementary Video 2 (MPG 1415 kb)
Supplementary Video 3 (MPG 1415 kb)
Supplementary Video 4 (MPG 1415 kb)
Supplementary Video 5 (MPG 1415 kb)
429_2013_683_MOESM6_ESM.tif
Supplementary Figure 1. Examples of diffusion ellipsoids prepared from the control group and from two individual rats in the SE and TBI groups. Note the region specific difference in the orientation (color of the ellipsoid) as well as axial and radial diffusivities (shape of the ellipsoid) between individual animals (TIFF 1239 kb)
Rights and permissions
About this article
Cite this article
Sierra, A., Laitinen, T., Gröhn, O. et al. Diffusion tensor imaging of hippocampal network plasticity. Brain Struct Funct 220, 781–801 (2015). https://doi.org/10.1007/s00429-013-0683-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0683-7