Abstract
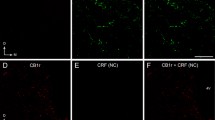
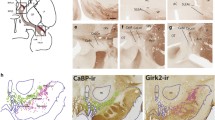
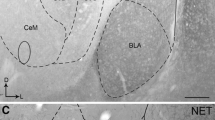
Amygdalar norepinephrine (NE) plays a key role in regulating neural responses to emotionally arousing stimuli and is involved in memory consolidation of emotionally charged events. Corticotropin-releasing factor (CRF) and dynorphin (DYN), two neuropeptides that mediate the physiological and behavioral responses to stress, are abundant in the central nucleus of the amygdala (CeA), and directly innervate brainstem noradrenergic locus coeruleus (LC) neurons. Whether the CRF- and DYN-containing amygdalar neurons receive direct noradrenergic innervation has not yet been elucidated. The present study sought to define cellular substrates underlying noradrenergic modulation of CRF- and DYN-containing neurons in the CeA using immunohistochemistry and electron microscopy. Ultrastructural analysis revealed that NE-labeled axon terminals form synapses with CRF- and DYN-containing neurons in the CeA. Semi-quantitative analysis showed that approximately 31 % of NET-labeled axon terminals targeted CeA neurons that co-expressed DYN and CRF. As a major source of CRF innervation to the LC, it is also not known whether CRF-containing CeA neurons are directly targeted by noradrenergic afferents. To test this, retrograde tract tracing using FluoroGold from the LC was combined with immunocytochemical detection of CRF and NET in the CeA. Our results revealed a population of LC-projecting CRF-containing CeA neurons that are directly innervated by NE afferents. Analysis showed that approximately 34 % of NET-labeled axon terminals targeted LC-projecting CeA neurons that contain CRF. Taken together, these results indicate significant interactions between NE, CRF and DYN in this critical limbic region and reveal direct synaptic interactions of NE with amygdalar CRF that influence the LC-NE arousal system.







Similar content being viewed by others
References
Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH et al (2008) Beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res 1209:65–73
Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207
Agnati LF, Zoli M, Stromberg I, Fuxe K (1995) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69(3):711–726
al-Damluji S (1988) Adrenergic mechanisms in the control of corticotrophin secretion. J Endocrinol 119:5–14
Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M et al (2013) Amygdala-dependent fear is regulated by oprl1 in mice and humans with PTSD. Sci Transl Med 5(188):188ra73. doi: 10.1126/scitranslmed.3005656
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999) The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12
Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH et al (1995) The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci 92:5062–5066
Asan E (1998) The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol 142:1–121
Aston-Jones G, Bloom FE (1981) Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1(887):900
Aston-Jones G, Chiang C, Alexinsky T (1991) Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res 88:501–520
Ballenger JC (2000) Anxiety and depression: optimizing treatments. Prim Care Companion J Clin Psychiatry 2:71–79
Benmansour S, Altamirano AV, Jones DJ, Sanchez TA, Gould GG, Pardon MJ et al (2004) Regulation of the norepinephrine transporter by chronic administration of antidepressants. Biol Psychiatry 55:313–316
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42:33–84
Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H (2004) Stress impairs a1A adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropharmacol 29:45–58
Bruchas MR, Land BB, Lemos JC, Chavkin C (2009) CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One 4(12):e8528. doi: 10.1371/journal.pone.0008528
Buckingham JC, Cooper TA (1986) Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinol 44(1):36–40
Buffalari DM, Grace AA (2007) Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci 27(45):12358–12366
Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463:235–272
Carvalho AF, Mackie K, Van Bockstaele EJ (2010) Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci 31:286–301
Cassell MD, Gray TS, Kiss JZ (1986) Neuronal architecture in the rat central nucleus of the amygdala: a cytological, histological, and immunocytochemical study. J Comp Neurol 246:478–499
Cecchi M, Khoshbouei H, Morilak DA (2002) Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacol 43(7):1139–1147
Chaijale NN, Curtis AL, Wood SK, Zhang XY, Bhatnagar S, Reyes BA et al (2013) Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharm 38(10):1833–1843
Chan J, Aoki C, Pickel VM (1990) Optimization of differential immunogold–silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods 33:113–127
Charney DS, Egnor RW (1989) Noradrenergic function in generalized anxiety disorder: effects of yohimbine in healthy subjects and patients with generalized anxiety disorder. Psychiatry Res 27:173–182
Chavkin C, James IF, Goldstein A (1982) Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215:413–415
Cole BJ, Koob GF (1988) Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther 247(3):902–910
Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ (1997) Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther 281:163–172
Curtis AL, Bello NT, Connally KR, Valentino RJ (2002) Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress termination. J Neuroendocrinol 14:667–682
Day HE, Campeau S, Watson SJ, Akil H (1997) Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat 13:115–139
De Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475
de la Perez Mora M, Jacobsen KX, Crespo-Ramirez M, Flores-Gracia C, Fuxe K (2008) Wiring and volume transmission in rat amygdala: implications for fear and anxiety. Neurochem Res 33(8):1618–1633
Descarries L, Mechawar N (2000) Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res 125:27–47
Domyancic AV, Morilak DA (1997) Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol 386(3):358–378
Duncan GE, Knapp DJ, Breese GR (1996) Neuroanatomical characterization of fos induction in rat behavioral models of anxiety. Brain Res 713:79–91
Dunn AJ, Berridge CW (1990) Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res 15:71–100
Dunn AJ, Swiergiel AH (2008) The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol 583(2):186–193
Dunn AJ, Swiergeil AH, Palamarchouk V (2004) Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann NY Acad Sci 1018:25–34
Erb S, Salmaso N, Rodaros D, Stewart J (2001) A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacol (Berl) 158(4):360–365
Fallon JH, Leslie FM (1986) Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol 249:293–336
Farb CR, Chang W, LeDoux JE (2010) Ultrastructural characterization of noradrenergic axons and beta-adrenergic receptors in the lateral nucleus of the amygdala. Front Behav Neurosci 4:162. doi: 10.3389/fnbeh.2010.00162
Farley IJ, Hornykiewicz O (1977) Noradrenaline distribution in subcortical areas of the human brain. Brain Res 126:53–62
Ferry B, Magistretti PJ, Pralong E (1997) Noradrenaline modulates glutamate-mediated neurotransmission in the rat basolateral amygdala in vitro. Eur J Neurosci 9:1356–1364
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914
Glass MJ, Colago EE, Pickel VM (2002) Alpha-2A-adrenergic receptors are present on neurons in the central nucleus of the amygdala that project to the dorsal vagal complex in the rat. Synapse 46(4):258–268
Gray TS (1993) Amygdaloid CRF pathways: role in autonomic, neuroendocrine, and behavioral responses to stress. Ann NY Acad Sci 697:53–60
Gray TS, Cassell MD, Kiss JZ (1984) Distribution of pro-opiomelanocortin-derived peptides and enkephalins in the rat central nucleus of the amygdala. Brain Res 306(1–2):354–358
Hatfield T, McGaugh JL (1999) Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem 71(2):232–239
Heinrichs SC, Pich EM, Miczek K, Britton KT, Koob GF (1992) Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res 581:190–197
Hopkins AL (2007) Network pharmacology. Nat Biotechnol 25(10):1110–1111
Jin J, Kittanakom S, Wong V, Reyes BAS, Van Bockstaele EJ, Stagljar I et al (2010) Interaction of the mu-opioid receptor with GPR177 (wntless) inhibits wnt secretion: potential implications for opioid dependence. BMC Neurosci 11:33
Kastenberger I, Lutsch C, Herzog H, Schwarzer C (2012) Influence of sex and genetic background on anxiety-related and stress-induced behaviour of prodynorphin-deficient mice. PLoS One 7(3):e34251
Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ et al (2009) Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry 14:37–50
Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J et al (2009) The global burden of mental disorders: an update from the WHO world mental health (WMH) surveys. Epidemiol Psychiatr Soc 18(1):23–33
Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA (2002) Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav 71(3):407–417
Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA (2007) Anxiolytic-like effects of k-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323:838–845
Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG et al (2011) k opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry 70:425–433
Koegler-Muly SM, Owens MJ, Ervin GN, Kilts CD, Nemeroff CB (1993) Potential corticotropin-releasing factor pathways in the rat brain as determined by bilateral electrolytic lesions of the central amygdaloid nucleus and the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 5:95–98
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharm 24(2):97–129
Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE et al (2013) Addiction as a stress surfeit disorder. Neuropharmacol. doi:10.1016/j.neuropharm.2013.05.024
Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ et al (2008) Presynpatic inhibition of diverse afferents to the locus coeruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 28:6516–6525
Krettek JE, Price JL (1978) A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178:255–280
Lam MP, Gianoulakis C (2011) Effects of acute ethanol on corticotropin-releasing hormone and β-endorphin systems at the level of the rat central amygdala. Psychopharmacol 218:229–239
Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008) The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28:407–414
LeDoux J (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Leranth C, Pickel VM (1989) Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborsky L (eds) Neuroanatomical tract-tracing methods II, recent progress. Plenum, New York, pp 129–172
Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M (1992) Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci 12:2313–2320
Lopez JF, Akil H, Watson SJ (1999) Neural circuits mediating stress. Biol Psychiatry 46:1461–1471
Marchant NJ, Densmore VS, Osborne PB (2007) Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol 504:702–715
McDonald AJ (1982) Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 208:401–418
McGaugh JL, McIntyre CK, Power AE (2002) Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem 78:539–552
McIntyre CK, Hatfield T, McGaugh JL (2002) Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci 16(7):1223–1226
McLaughlin JP, Marton-Popovici M, Chavkin C (2003) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23:5674–5683
Merali Z, McIntosh J, Kent P, Michaud D, Anisman H (1998) Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 18:4758–4766
Merchenthaler I, Maderdrut JL, Cianchetta P, Shughrue P, Bronstein D (1997) In situ hybridization histochemical localization of prodynorphin messenger RNA in the central nervous system of the rat. J Comp Neurol 384:211–232
Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF (1995) Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 15:5439–5447
Moga MM, Saper CB, Gray TS (1990) Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. J Comp Neurol 295:662–682
Nikolarakis KE, Almeida OFX, Herz A (1986) Stimulation of hypothalamic β-endorphin and dynorphin release by corticotropin-releasing factor (in vitro). Brain Res 399(1):152–155
Pacak K, Palkovits M, Kvetnansky R, Fukuhara K, Armando I, Kopin IJ et al (1993) Effects of single or repeated immobilization on release of norepinephrine and its metabolites in the central nucleus of the amygdala in conscious rats. Neuroendocrinol 57(4):626–633
Page ME, Abercrombie ED (1999) Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synpase 33:304–313
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, 3rd edn. Academic Press Inc, San Diego
Petrovich GD, Canteras NS, Swanson LW (2001) Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev 38:247–289
Pich EM, Koob GF, Sattler SC, Menzaghi F, Heilig M, Heinrichs SC et al (1992) Stress-induced release of corticotropin-releasing factor in the amygdala measured by in vivo microdialysis. Neurosci Abstr 18:535
Plotsky PM, Cunningham ETJ, Widmaier EP (1989) Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev 10:437–458
Quirarte GL, Galvez R, Roozendaal B, McGaugh JL (1998) Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res 808:134–140
Raber J, Koob GF, Bloom FE (1995) Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling; comparison with the hypothalamic response. J Pharmacol Exp Ther 272(2):815–824
Retson TA, Van Bockstaele EJ (2013) Coordinate regulation of noradrenergic and serotonergic brain regions by amygdalar neurons. J Chem Neuroanat 52:9–19
Reyes BA, Van Bockstaele EJ (2007) Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res 1117(1):69–79
Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ (2005) Hypothalamic projections to the locus coeruleus neurons in rat brain. Eur J Neurosci 22:93–106
Reyes BAS, Johnson AD, Glaser JD, Commons KG, Van Bockstaele EJ (2007) Dynorphin-containing axons directly innervate noradrenergic neurons in the rat nucleus locus coeruleus. Neuroscience 145:1077–1086
Reyes BA, Valentino RJ, Van Bockstaele EJ (2008) Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology 149:122–130
Reyes BAS, Carvalho AF, Vakharia K, Van Bockstaele EJ (2011) Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Exp Neurol 230(1):96–105
Reyes BA, Vakharia K, Ferraro TN, Levenson R, Berrettini WH, Van Bockstaele EJ (2012) Opiate agonist-induced re-distribution of wntless, a mu-opioid receptor interacting protein, in rat striatal neurons. Exp Neurol 233:205–213
Rivier J, Spiess J, Vale W (1983) Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci 80:4851–4855
Roder S, Ciriello J (1993) Innervation of the amygdaloid complex by catecholaminergic cell groups of the ventrolateral medulla. J Comp Neurol 332:105–122
Rodriguez de Fonseca F, Carrera MRA, Navarro M, Koob GF, Weiss F (1997) Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276:2050–2054
Ronan P, Summers C (2011) Molecular signaling and translational significance of the corticotropin releasing factor system. Prog Mol Bio Transl Sci 98:235–292
Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG et al (1996) Distribution of alpha 2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol 372(1):135–165
Rudoy CA, Van Bockstaele EJ (2005) Cocaine effects on norepinephrine in the amygdala: Cocaine withdrawal-related anxiety and stress-related relapse. Cell sci rev 2
Rudoy CA, Reyes AR, Van Bockstaele EJ (2009) Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal. Neuropsychopharm 34(5):1135–1148
Sah P, Faber ES, De Lopez Armentia M, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83:803–834
Sakanaka M, Shibasaki T, Lederis K (1986) Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res 382(2):213–238
Sawada K, Fukui Y, Hawkes R (2008) Spatial distribution of corticotropin-releasing factor immunopositive climbing fibers in the mouse cerebellum: analysis by whole mount immunohistochemistry. Brain Res 1222:106–117
Sawchenko PE, Swanson LW, Vale WW (1984) Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci 81:1883–1887
Schneier FR (2011) Pharmacotherapy of social anxiety disorder. Expert Opin Pharmacother 12:615–625
Schroeter S, Appasundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD (2000) Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol 420:211–232
Seguela P, Watkins KC, Geffard M, Descarries L (1990) Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neuroscience 35:249–264
Shippenberg TS, Zapata A, Chefer VI (2007) Dynorphin and pathophysiology of drug addiction. Pharmacol Ther 116:306–321
Smith HR, Beveridge TJR, Porrino LJ (2006) Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience 138:703–714
Somers JM, Goldner EM, Waraich P, Hsu L (2006) Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Canadian J Psychiatry 51:100–113
Stone EA, Zhang Y, Hiller JM, Simon EJ, Hillman DE (1997) Activation of fos in mouse amygdala by local infusion of norepinephrine or atipamezole. Brain Res 778(1):1–5
Swanson L, Sawchenko P, Rivier J, Vale W (1983) The organization of ovine corticotropin releasing factor (CRF)-immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinol 36:165–186
Swiergiel AH, Takahashi LK, Kalin NH (1993) Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res 623:229–234
Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR (1996) Distribution of alpha 2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol 372(1):111–134
Tejani-Butt SM (1992) [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther 260:427–436
Valentino RJ, Foote SL, Page ME (1993) The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann NY Acad Sci 697:173–188
Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ (2001) Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience 106(2):375–384
Van Bockstaele EJ, Colago EE, Valentino RJ (1996) Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol 364:523–534
Van Bockstaele EJ, Colago EE, Valentino RJ (1998) Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol 10:743–757
Van Bockstaele EJ, Reyes BA, Valentino RJ (2010) The locus coeruleus: a key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res 1314:162–174
Veening JG, Swanson LW, Sawchenko PE (1984) The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res 303:337–357
Wee S, Koob GF (2010) The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacol (Berl) 210:121–135
Williams CL, Men D, Clayton EC, Gold PE (1998) Norepinephrine release in the amygdala following systemic injection of epinephrine or escapable foot shock: contribution of the nucleus of the solitary tract. Behav Neurosci 112:1414–1422
Wittchen HU, Jacobi F (2005) Size and burden of mental disorders in Europe: a critical review and appraisal of 27 studies. Eur Neuropsychopharmocol 15:357–376
Wittmann W, Eduard SE, Rosskothen I, Gaburro S, Singewald N, Herzog H et al (2009) Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacol 34:775–785
Yi H, Leunissen J, Ge-Ming S, Gutekunst C, Hersch S (2001) A novel procedure for pre-embedding double immunogold–silver labeling at the ultrastructural level. J Histochem Cytochem 49(3):279–283
Zardetto-Smith AM, Gray TS (1990) Organization of peptidergic and catecholaminergic efferents from the nucleus of the solitary tract to the rat amygdala. Brain Res Bull 25(6):875–887
Zhang JJ, Swiergiel AH, Palamarchouk VS, Dunn AJ (1998) Intracerebroventricular infusion of CRF increases extracellular concentrations of norepinephrine in the hippocampus and cortex as determined by in vivo voltammetry. Brain Res Bull 47(3):277–284
Zhang J, Muller JF, McDonald AJ (2013) Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. J Neurosci 228:395–408
Acknowledgments
This project was supported by the National Institutes of Health grants DA009082 to E.J.V.B. and DA018326 to E.M.U. We acknowledge the experimental contributions of Mr. Nathan Heldt.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kravets, J.L., Reyes, B.A.S., Unterwald, E.M. et al. Direct targeting of peptidergic amygdalar neurons by noradrenergic afferents: linking stress-integrative circuitry. Brain Struct Funct 220, 541–558 (2015). https://doi.org/10.1007/s00429-013-0674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0674-8