Abstract
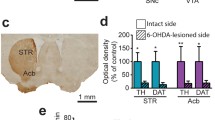
Using transgenic mice that express enhanced green fluorescent protein (EGFP) under the control of the tyrosine hydroxylase (TH) promoter, we have previously shown that there are approximately 3,000 striatal EGFP-TH interneurons per hemisphere in mice. Here, we report that striatal TH-EGFP interneurons exhibit a small, transient but significant increase in number after unilateral destruction of the nigrostriatal dopaminergic pathway. The increase in cell number is accompanied by electrophysiological and morphological changes. The intrinsic electrophysiological properties of EGFP-TH interneurons ipsilateral to 6-OHDA lesion were similar to those originally reported in intact mice except for a significant reduction in the duration of a characteristic depolarization induced plateau potential. There was a significant change in the distribution of the four previously described electrophysiologically distinct subtypes of striatal TH interneurons. There was a concomitant increase in the frequency of both spontaneous excitatory and inhibitory post-synaptic currents, while their amplitudes did not change. Nigrostriatal lesions did not affect somatic size or dendritic length or branching, but resulted in an increase in the density of proximal dendritic spines and spine-like appendages in EGFP-TH interneurons. The changes indicate that electrophysiology properties and morphology of striatal EGFP-TH interneurons depend on endogenous levels of dopamine arising from the nigrostriatal pathway. Furthermore, these changes may serve to help compensate for the changes in activity of spiny projection neurons that occur following loss of the nigrostriatal innervation in experimental or in early idiopathic Parkinson’s disease by increasing feedforward GABAergic inhibition exerted by these interneurons.











Similar content being viewed by others
References
Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT (1997) Dopaminergic neurons intrinsic to the primate striatum. J Neurosci 17:6761–6768
Breese GR, Traylor TD (1971) Depletion of brain noradrenaline and dopamine by 6-hydroxydopamine. Br J Pharmacol 42:88–99
Busceti CL, Biagioni F, Mastroiacovo F, Bucci D, Lenzi P, Pasquali L, Trabucco A, Nicoletti F, Fornai F (2008) High number of striatal dopaminergic neurons during early postnatal development: correlation analysis with dopaminergic fibers. J Neural Transm 115:1375–1383
Busceti CL, Bucci D, Molinaro G, Di Pietro P, Zangrandi L, Gradini R, Moratalla R, Battaglia G, Bruno V, Nicoletti F, Fornai F (2012) Lack or inhibition of dopaminergic stimulation induces a development increase of striatal tyrosine hydroxylase-positive interneurons. PLoS One 7(9):e44025
Bustos G, Abarca J, Campusano J, Bustos V, Noriega V, Aliaga E (2004) Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain. Brain Res Brain Res Rev 47:126–144
Calabresi P, Centonze D, Bernardi G (2000) Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci 23:S57–S63
Cenci MA, Lundblad M (2007) Ratings of l-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr Protoc Neurosci Chapter 9:Unit 9.25
Darmopil S, Muñetón-Gómez VC, de Ceballos ML, Bernson M, Moratalla R (2008) Tyrosine hydroxylase cells appearing in the mouse striatum after dopamine denervation are likely to be projection neurones regulated by l-DOPA. Eur J Neurosci 27:580–592
Dehorter N, Guigoni C, Lopez C, Hirsch J, Eusebio A, Ben-Ari Y, Hammond C (2009) Dopamine-deprived striatal GABAergic interneurons burst and generate repetitive gigantic IPSCs in medium spiny neurons. J Neurosci 29:7776–7787
Dejean C, Nadjar A, Le Moine C, Bioulac B, Gross CE, Boraud T (2012) Evolution of the dynamic properties of the cortex-basal ganglia network after dopaminergic depletion in rats. Neurobiol Dis 46:402–413
Dubach M, Schmidt R, Kunkel D, Bowden DM, Martin R, German DC (1987) Primate neostriatal neurons containing tyrosine hydroxylase: immunohistochemical evidence. Neurosci Lett 75:205–210
English DF, Ibáñez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koós T (2011) GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci 15:123–130
Fino E, Glowinski J, Venance L (2007) Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci Res 58:305–316
Franklin KBJ, Paxinos G (2007) The mouse brain in stereotaxic coordinates, 3rd edn. Academic Press, San Diego
Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC (2010) Distinct roles of GABAergic interneuron in the regulation of striatal output pathways. J Neurosci 30:2223–2234
Gittis AH, Hang GB, LaDow ES, Shoenfeld LR, Atallah BV, Finkbeiner S, Kreitzer AC (2011) Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron 71:858–868
Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925
Gustafson N, Gireesh-Dharmaraj E, Czubayko U, Blackwell KT, Plenz D (2006) A comparative voltage and current-clamp analysis of feedback and feedforward synaptic transmission in the striatal microcircuit in vitro. J Neurophysiol 95:737–752
Henny P, Brown MT, Northrop A, Faunes M, Ungless MA, Magill PJ, Bolam JP (2012) Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci 15:613–619
Huot P, Parent A (2007) Dopaminergic neurons intrinsic to the striatum. J Neurochem 101:1441–1447
Huot P, Levesque M, Parent A (2007) The fate of striatal dopaminergic neurons in Parkinson’s disease and Huntington’s chorea. Brain 130:222–232
Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T, Tepper JM (2011) A novel functionally distinct subtype of striatal neuropeptide Y interneuron. J Neurosci 31:16757–16769
Ibáñez-Sandoval O, Tecuapetla F, Ünal B, Shah F, Koós T, Tepper JM (2010) Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci 30:6999–7016
Jollivet C, Montero-Menei CN, Venier-Julienne MC, Sapin A, Benoit JP, Menei P (2004) Striatal tyrosine hydroxylase immunoreactive neurons are induced by l-dihydroxyphenylalanine and nerve growth factor treatment in 6-hydroxydopamine lesioned rats. Neurosci Lett 362:79–82
Kawaguchi Y (1993) Physiological, morphological and histochemical characterisation of three classes of interneurons in the neostriatum. J Neurosci 13:4908–4923
Kawaguchi Y (1997) Neostriatal cell subtypes and their functional roles. Neurosci Res 27:1–8
Kita H, Kita T (2011) Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. J Neurosci 31:10311–10322
Koós T, Tepper JM (1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2:467–472
Koós T, Tepper JM, Wilson CJ (2004) Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci 24:7916–7922
Lee CR, Tepper JM (2007) A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci 27:6531–6541
Lopez-Real A, Rodriguez-Pallares J, Guerra MH, Labandeira-Garcia JL (2003) Localization and functional significance of striatal neurons immunoreactive to aromatic l-amino acid decarboxylase or tyrosine hydroxylase in rat parkinsonian models. Brain Res 969:135–146
Luo R, Schroeder MJ, Partridge JG, Vicini S (2012) Direct and GABA mediated indirect effects of nicotinic ACh receptor agonists on striatal neurones. J Physiol 591:203–207
Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P (2000) Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol 524(1):91–116
Masuda M, Miura M, Inoue R, Imanishi M, Saino-Saito S, Takada M, Kobayashi K, Aosaki T (2011) Postnatal development of tyrosine hydroxylase mRNA-expressing in mouse neostriatum. Eur J Neurosci 34(9):1355–1367
Mathus BN, Capik NA, Alvarez VA, Lovinger DM (2011) Serotonin induces long-term depression at corticostriatal synapses. J Neurosci 31(20):7402–7411
Mazloom M, Smith Y (2006) Synaptic microcircuitry of tyrosine hydroxylase-containing neurons and terminals in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treated monkeys. J Comp Neurol 495:453–469
Meredith GE, Farrell T, Kellaghan P, Tan Y, Zahm DS, Toterdell S (1999) Immunocytochemical characterization of catecholaminergic neurons in the rat striatum following dopamine-depleting lesions. Eur J Neurosci 11:3583–3596
Palfi S, Leventhal L, Chu Y, Ma SY, Emborg M, Bakay R, Deglon N, Hantraye P, Aebischer P, Kordower JH (2002) Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J Neurosci 22:4942–4954
Porritt MJ, Batchelor PE, Hughes AJ, Kalnins R, Donnan GA, Howells DW (2000) New dopaminergic neurons in Parkinson’s disease striatum. Lancet 356:44–45
Porritt MJ, Kingsbury AE, Hughes AJ, Howells DW (2006) Striatal dopaminergic neurons are lost with Parkinson’s disease progression. Mov Disord 21:2208–2211
Raju DV, Ahem TH, Wright TM, Standaert DG, Hall RA, Smith Y (2008) Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of Parkinsonism. Eur J Neurosci 27:1647–1658
Rymar VV, Sasseville R, Luk KC, Sadikot AF (2004) Neurogenesis and stereological morphometry of calretinin immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol 469:325–339
San Sebastián W, Guillén J, Manrique M, Belzunegui S, Ciordia E, Izal-Azcárate A, Garrido-Gil P, Vázquez-Claverie M, Luquin MR (2007) Modification of the number and phenotype of striatal dopaminergic cells by carotid body graft. Brain 130:1306–1316
Schultz W, Ungerstedt U (1978) Short-term increase and long-term reversion of striatal cell activity after degeneration of the nigrostriatal dopamine system. Exp Brain Res 33:159–171
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87(4):387–406
Tandé D, Hoglinger G, Debeir T, Freundlieb N, Hirsch EC, Francois C (2006) New striatal dopamine neurons in MPTP-treated macaques result from a phenotypic shift and not neurogenesis. Brain 129:1194–1200
Tashiro Y, Sugimoto T, Hattori T, Uemura Y, Nagatsu I, Kikuchi H, Mizuno N (1989) Tyrosine hydroxylase-like immunoreactive neurons in the striatum of the rat. Neurosci Lett 97:6–10
Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–692
Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4:150. doi:10.3389/fnana.2010.00150
Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG (2001) Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci 21:6430–6439
Ugrumov MV (2009) Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J Chem Neuroanat 38:241–256
Ünal B, Ibáñez-Sandoval O, Shah F, Abercrombie ED, Tepper JM (2011) Distribution of tyrosine hydroxylase-expressing interneurons with respect to anatomical organization of the neostriatum. Front Syst Neurosci 5:41
Ungless MA, Magill PJ, Bolam JP (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303:2040–2042
Wilson CJ, Kawaguchi Y (1996) The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16:2397–2410
Wu Y, Parent A (2000) Striatal interneurons expressing calretinin, parvalbumin and NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain Res 863:182–191
Xenias H, Shah F, Koós T, Tepper JM (2012) Voltammetric studies of dopamine release from optogenetically activated striatal TH+ interneurons. Soc Neurosci Abstr 183.12/LL3
Yamashita T, Isa T (2003) Flufenamic acid sensitive, Ca(2+)-dependent inward current induced by nicotinic acetylcholine receptors in dopamine neurons. Neurosci Res 46:463–473
Zackheim J, Abercrombie ED (2005) Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience 131:423–436
Acknowledgments
This research was supported by National Institutes of Health Grant NINDS-NS034865 (J.M.T.) and Rutgers University. We thank Dr. Elizabeth D. Abercrombie for generously allowing us the use of microscope and image acquisition and analysis software for stereology and neuron reconstruction. We thank Drs. Elizabeth D. Abercrombie, Çağrı T. Ünal and Osvaldo Ibáñez-Sandoval for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ünal, B., Shah, F., Kothari, J. et al. Anatomical and electrophysiological changes in striatal TH interneurons after loss of the nigrostriatal dopaminergic pathway. Brain Struct Funct 220, 331–349 (2015). https://doi.org/10.1007/s00429-013-0658-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0658-8