Abstract
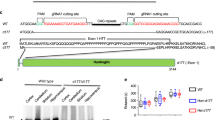
Huntington’s disease (HD) is a genetically neurodegenerative disease, affecting the central nervous system and leading to mental and motor dysfunctions. To date, there is no cure for HD; as a result, HD patients gradually suffer devastating symptoms, such as chorea, weight loss, depression and mood swings, until death. According to previous studies, the exon 1 region of the huntingtin (HTT) gene with expanded CAG trinucleotide repeats plays a critical role in causing HD. In vitro studies using exon 1 of HTT fused with green fluorescent protein (GFP) gene have facilitated discovering several mechanisms of HD. However, whether this chimera construct exerts similar functions in vivo is still not clear. Here, we report the generation of transgenic mice carrying GFP fused with mutant HTT exon 1 containing 84 CAG trinucleotide repeats, and the evaluation of phenotypes via molecular, neuropathological and behavioral analyses. Results show that these transgenic mice not only displayed neuropathological characteristics, observed either by green fluorescent signals or by immunohistochemical staining, but also progressively developed pathological and behavioral symptoms of HD. Most interestingly, these transgenic mice showed significantly differential expression levels of nuclear aggregates between cortex and striatum regions, highly mimicking selective expression of mutant HTT in HD patients. To the best of our knowledge, this is the first report showing different nuclear diffusion profiling in mouse models with transgenic mice carrying the exon 1 region of mutant HTT. Our model will be beneficial for tracing the expression of mutant HTT and accelerating the understanding of selective pathological progression in HD.






Similar content being viewed by others
References
Carter RJ, Lione LA, Humby T et al (1999) Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci 19:3248–3257
Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263:802–805
Chan AW, Yang SH (2009) Generation of transgenic monkeys with human inherited genetic disease. Methods 49:78–84
Chiang MC, Chern Y, Juo CG (2011) The dysfunction of hepatic transcriptional factors in mice with Huntington’s Disease. Biochim Biophys Acta 1812:1111–1120
Ciammola A, Sassone J, Sciacco M et al (2011) Low anaerobic threshold and increased skeletal muscle lactate production in subjects with Huntington’s disease. Mov Disord 26:130–137
Cornett J, Cao F, Wang CE et al (2005) Polyglutamine expansion of huntingtin impairs its nuclear export. Nat Genet 37:198–204
Davies SW, Turmaine M, Cozens BA et al (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90:537–548
DiFiglia M, Sapp E, Chase K et al (1995) Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14:1075–1081
Hodgson JG, Agopyan N, Gutekunst CA et al (1999) A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23:181–192
Hogan B (1994) Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Li S, Li XJ (2006) Multiple pathways contribute to the pathogenesis of Huntington disease. Mol Neurodegener 1:19
Lin CH, Tallaksen-Greene S, Chien WM et al (2001) Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Hum Mol Genet 10:137–144
Maat-Schieman M, Roos R, Losekoot M et al (2007) Neuronal intranuclear and neuropil inclusions for pathological assessment of Huntington’s disease. Brain Pathol 17:31–37
Mangiarini L, Sathasivam K, Seller M et al (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506
McGuire JR, Rong J, Li SH, Li XJ (2006) Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem 281:3552–3559
Moffitt H, McPhail GD, Woodman B, Hobbs C, Bates GP (2009) Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of Huntington’s disease. PLoS ONE 4:e8025
Schilling G, Becher MW, Sharp AH et al (1999) Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet 8:397–407
The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
von Horsten S, Schmitt I, Nguyen HP et al (2003) Transgenic rat model of Huntington’s disease. Hum Mol Genet 12:617–624
Walker FO (2007) Huntington’s disease. Lancet 369:218–228
Wang CE, Tydlacka S, Orr AL et al (2008) Accumulation of N-terminal mutant huntingtin in mouse and monkey models implicated as a pathogenic mechanism in Huntington’s disease. Hum Mol Genet 17:2738–2751
Williams A, Sarkar S, Cuddon P et al (2008) Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol 4:295–305
Yang SH, Chan AW (2011) Transgenic animal models of Huntington’s Disease. Curr Top Behav Neurosci 7:61–85
Yang SH, Cheng PH, Banta H et al (2008) Towards a transgenic model of Huntington’s disease in a non-human primate. Nature 453:921–924
Acknowledgments
We thank Jonathan Courtenay for critical reading of the manuscript, Dr. Xiao-Jiang Li for providing mEM48 antibodies, Dr. Chauying Jen and Pi-Hsueh Shirley Li for providing equipment and Dr. Shaw-Jeng Tsai and Dr. H. Sunny Sun for support and suggestions. This work was supported by National Science Council grants (NSC 99-2320-B-006-026-MY3 and NSC 100-2627-B-006-023) and in part by grant of the Ministry of Education, Taiwan, Republic of China, under the ATU plan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
P.-H. Cheng and C.-L. Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
429_2012_401_MOESM1_ESM.tif
Online resource 1 Copy numbers of transgene in Ubi-HTT84Q transgenic founders. Copy numbers of transgene in different transgenic founders were determined by using Southern blotting. Top panel shows the original image of Southern blotting, and numbers listed above represent the tag number of different transgenic founders. The bottom table shows the estimated copy numbers of each transgenic founder based on the Southern blotting result. (TIFF 2975 kb)
429_2012_401_MOESM2_ESM.tif
Online resource 2 Neuropathological phenotypes of HD in the brain of Ubi-G-HTT84Q transgenic mice at 2 and 6 months of age. Brain sections were sampled from Ubi-G-HTT84Q transgenic mice at 2 and 6 months of age, and stained with the mEM48 antibody via DAB immunohistochemistry staining. (a-c) Representative brain sections from Ubi-G-HTT84Q transgenic mice at 2 months of age. (d-f) Representative brain sections from Ubi-G-HTT84Q transgenic mice at 6 months of age. There are no aggregates in striatum (STR), cortex (CTX) and white matter (WM) regions at 2 months of age; however, weaker signals of neuropil aggregates are observed at 6 months of age as indicated in arrows. (TIFF 3268 kb)
429_2012_401_MOESM3_ESM.tif
Online resource 3 No neuropathological phenotypes of HD in the brains of non-transgenic littermates at 12 months of age. Brain sections were sampled from non-transgenic littermates at 12 months of age, and stained with the mEM48 antibody via DAB immunohistochemistry staining. (a) Lower magnification shows no aggregates in striatum (STR), cortex (CTX) and white matter (WM) regions. (b) A higher power image of STR displays no aggregates. (c) A higher power image shows no aggregates in CTX. (TIFF 1598 kb)
429_2012_401_MOESM4_ESM.tif
Online resource 4 Neuropathological phenotypes of HD in the brain of Ubi-G-HTT84Q transgenic mice Brain sections were sampled from one representative transgenic mouse at 12 months of age, and stained with the mEM48 antibody via DAB immunohistochemistry staining. Nucleus staining was performed using hematoxylin. (a) STR displays nuclear aggregates, intranuclear aggregates and neuropil aggregates. (b) Neuropil aggregates are dominant in CTX. Arrow heads indicate nuclear aggregates, arrows indicate intranuclear aggregates and stars indicate neuropil aggregates. (TIFF 2424 kb)
429_2012_401_MOESM5_ESM.tif
Online resource 5 Behavioral phenotypes of Ubi-G-HTT84Q transgenic mice before the onset of HD. (a) Footprinting (top panel) and rota rod (bottom panel) tests at 4 months of age. (b) Footprinting (top panel) and rota rod (bottom panel) tests at 6 months of age. There are no statistical difference (p > 0.05) between Ubi-G-HTT84Q transgenic mice (GHD) and non-transgenic mice (WT). Data represented mean ± SD. (TIFF 3064 kb)
429_2012_401_MOESM6_ESM.tif
Online resource 6 Genotyping of Ubi-G-HTT19Q and Ubi-G-HTT84Q transgenic mice. PCR with specific primers was used to confirm the transgenic status. 8-1 – 5 are Ubi-G-HTT19Q transgenic mice showing shorter PCR amplicons, whereas 2-1 – 5 are Ubi-G-HTT84Q transgenic mice showing longer amplicons due to longer CAG repeats. (TIFF 1158 kb)
429_2012_401_MOESM7_ESM.tif
Online resource 7 Neuropathological phenotypes of HD in the brain of Ubi-HTT84Q transgenic mice. Brain sections were sampled from one representative transgenic mouse at 6 months of age, and stained with the mEM48 antibody via DAB immunohistochemistry staining. Images show more nuclear aggregates (small spots) in striatum (STR) and cortex (CTX) region than in white matter region (WM). (TIFF 3597 kb)
Rights and permissions
About this article
Cite this article
Cheng, PH., Li, CL., Her, LS. et al. Significantly differential diffusion of neuropathological aggregates in the brain of transgenic mice carrying N-terminal mutant huntingtin fused with green fluorescent protein. Brain Struct Funct 218, 283–294 (2013). https://doi.org/10.1007/s00429-012-0401-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0401-x