Abstract
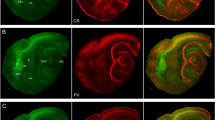
This study describes the organisation of the entorhinal cortex of the Megachiroptera, straw-coloured fruit bat and Wahlberg’s epauletted fruit bat. Using Nissl and Timm stains, parvalbumin and SMI-32 immunohistochemistry, we identified five fields within the medial (MEA) and lateral (LEA) entorhinal areas. MEA fields E CL and E C are characterised by a poor differentiation between layers II and III, a distinct layer IV and broad, stratified layers V and VI. LEA fields E I, E R and E L are distinguished by cell clusters in layer II, a clear differentiation between layers II and III, a wide columnar layer III and a broad sublayer Va. Clustering in LEA layer II was more typical of the straw-coloured fruit bat. Timm-staining was most intense in layers Ib and II across all fields and layer III of field E R. Parvalbumin-like staining varied along a medio-lateral gradient with highest immunoreactivity in layers II and III of MEA and more lateral fields of LEA. Sparse SMI-32-like immunoreactivity was seen only in Wahlberg’s epauletted fruit bat. Of the neurons in MEA layer II, ovoid stellate cells account for ~38%, polygonal stellate cells for ~8%, pyramidal cells for ~18%, oblique pyramidal cells for ~6% and other neurons of variable morphology for ~29%. Differences between bats and other species in cellular make-up and cytoarchitecture of layer II may relate to their three-dimensional habitat. Cytoarchitecture of layer V in conjunction with high encephalisation and structural changes in the hippocampus suggest similarities in efferent hippocampal → entorhinal → cortical interactions between fruit bats and primates.









Similar content being viewed by others
Abbreviations
- EC:
-
Entorhinal cortex
- MEA:
-
Medial entorhinal cortex
- LEA:
-
Lateral entorhinal cortex
- PrS:
-
Presubiculum
- PaS:
-
Parasubiculum
- PRh:
-
Perirhinal cortex
- POR:
-
Postrhinal cortex
- PPC:
-
Prepiriform cortex
- E CL :
-
Caudal-limiting entorhinal field
- E C :
-
Caudal entorhinal field
- E I :
-
Intermediate entorhinal field
- E L :
-
Lateral entorhinal field
- E R :
-
Rostral entorhinal field
References
Alonso A, Klink R (1993) Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol 70:128–143
Alonso A, Llinas RR (1989) Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342:175–177
Amaral DG, Insausti R, Cowan WM (1987) The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurol 264:326–355
Ashwell KW, Zhang LL, Marotte LR (2005) Cyto- and chemoarchitecture of the cortex of the tammar wallaby (Macropus eugenii): areal organization. Brain Behav Evol 66:114–136
Baron G, Stephan H, Frahm HD (1996a) Atlases of a Megachiroptera brain. In: Baron G, Stephan H, Frahm HD (eds) Comparative neurobiology of Chiroptera: macromorphology, brain structures, tables and atlases, vol 1. Birkhäuser Verlag, Basel, pp 433–529
Baron G, Stephan H, Frahm HD (1996b) Comparative brain characteristics. In: Baron G, Stephan H, Frahm HD (eds) Comparative neurobiology of Chiroptera: macromorphology, brain structures, tables and atlases, vol 1. Birkhäuser Verlag, Basel, 529 pp
Beall MJ, Lewis DA (1992) Heterogeneity of layer II neurons in human entorhinal cortex. J Comp Neurol 321:241–266
Blaizot X, Martinez-Marcos A, Arroyo-Jimenez MdM, Marcos P, Artacho-Perula E, Munoz M, Chavoix C, Insausti R (2004) The parahippocampal gyrus in the Baboon: anatomical, cytoarchitectonic and magnetic resonance imaging (MRI) Studies. Cereb Cortex 14:231–246
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig
Brodmann K (1925) Vergleichende Lokalisationslehre der Grosshirnrinde. C. G. Leipzig, Röder GmbH, pp 177–183
Brun VH, Solstad T, Kjelstrup KB, Fyhn M, Witter MP, Moser EI, Moser MB (2008) Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18:1200–1212
Buhl EH, Dann JF (1991) Cytoarchitecture, neuronal composition, and entorhinal afferents of the flying fox hippocampus. Hippocampus 1:131–152
Burwell RD, Amaral DG (1998) Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol 391:293–321
Campbell MJ, Morrison JH (1989) Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol 282:191–205
DeFelipe J, Hendry SH, Jones EG (1989) Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc Natl Acad Sci USA 86:2093–2097
Dorph-Petersen KA, Nyengaard JR, Gundersen HJG (2001) Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc 204:232–246
Erchova I, Kreck G, Heinemann U, Herz AV (2004) Dynamics of rat entorhinal cortex layer II and III cells: characteristics of membrane potential resonance at rest predict oscillation properties near threshold. J Physiol 560:89–110
Fonseca M, Soriano E, Ferrer I, Martinez A, Tunon T (1993) Chandelier cell axons identified by parvalbumin-immunoreactivity in the normal human temporal cortex and in Alzheimer’s disease. Neuroscience 55:1107–1116
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B (2004) Spatial representation in the entorhinal cortex. Science 305:1258–1264
Fyhn M, Hafting T, Witter MP, Moser EI, Moser M-B (2008) Grid cells in mice. Hippocampus 18:1230–1238
Garden DL, Dodson PD, O’Donnell C, White MD, Nolan MF (2008) Tuning of synaptic integration in the medial entorhinal cortex to the organization of grid cell firing fields. Neuron 60:875–889
Geneser-Jensen FA, Haug FM, Danscher G (1974) Distribution of heavy metals in the hippocampal region of the guinea pig. A light microscope study with Timm’s sulfide silver method. Z Zellforsch Mikrosk Anat 147:441–478
Germroth P, Schwerdtfeger WK, Buhl EH (1989a) GABAergic neurons in the entorhinal cortex project to the hippocampus. Brain Res 494:187–192
Germroth P, Schwerdtfeger WK, Buhl EH (1989b) Morphology of identified entorhinal neurons projecting to the hippocampus. A light microscopical study combining retrograde tracing and intracellular injection. Neuroscience 30:683–691
Germroth P, Schwerdtfeger WK, Buhl EH (1991) Ultrastructure and aspects of functional organization of pyramidal and nonpyramidal entorhinal projection neurons contributing to the perforant path. J Comp Neurol 305:215–231
Giocomo LM, Hasselmo ME (2008) Time constants of h current in layer ii stellate cells differ along the dorsal to ventral axis of medial entorhinal cortex. J Neurosci 28:9414–9425
Goldstein ME, Sternberger LA, Sternberger NH (1983) Microheterogeneity (“neurotypy”) of neurofilament proteins. Proc Natl Acad Sci USA 80:3101–3105
Gundersen HJG, Jensen EB, Kiêu K, Nielsen J (1999) The efficiency of systematic sampling in stereology–reconsidered. J Microsc 193:199–211
Hafting T, Fyhn M, Molden S, Moser MB, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806
Hamam BN, Amaral DG, Alonso AA (2000) Morphological and electrophysiological characteristics of layer V neurons of the rat medial entorhinal cortex. J Comp Neurol 418:457–472
Hamam BN, Amaral DG, Alonso AA (2002) Morphological and electrophysiological characteristics of layer V neurons of the rat lateral entorhinal cortex. J Comp Neurol 451:45–61
Hassiotis M, Paxinos G, Ashwell KW (2004) Cyto- and chemoarchitecture of the cerebral cortex of the Australian echidna (Tachyglossus aculeatus). I. Areal organization. J Comp Neurol 475:493–517
Hassiotis M, Paxinos G, Ashwell KW (2005) Cyto- and chemoarchitecture of the cerebral cortex of an echidna (Tachyglossus aculeatus). II. Laminar organization and synaptic density. J Comp Neurol 482:94–122
Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P (1989) Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res 76:467–472
Hof P, Sherwood C (2005) Morphomolecular neuronal phenotypes in the neocortex reflect phylogenetic relationships among certain mammalian orders. Anat Rec A Discov Mol Cell Evol Biol 287A:1153–1163
Hof PR, Cox K, Morrison JH (1990) Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol 301:44–54
Hof PR, Glezer II, Nimchinsky EA, Erwin JM (2000) Neurochemical and cellular specializations in the mammalian neocortex reflect phylogenetic relationships: evidence from primates, cetaceans, and artiodactyls. Brain Behav Evol 55:300–310
Holm IE, Geneser FA (1989) Histochemical demonstration of zinc in the hippocampal region of the domestic pig: I. Entorhinal area, parasubiculum, and presubiculum. J Comp Neurol 287:145–163
Iñiguez C, Gayoso MJ, Carreres J (1985) A versatile and simple method for staining nervous tissue using Giemsa dye. J Neurosci Methods 13:77–86
Insausti R, Tuñón T, Sobreviela T, Insausti AM, Gonzalo LM (1995) The human entorhinal cortex: a cytoarchitectonic analysis. J Comp Neurol 355:171–198
Insausti R, Herrero MT, Witter MP (1997) Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus 7:146–183
Jeffery KJ (2007) Integration of the sensory inputs to place cells: what, where, why, and how? Hippocampus 17:775–785
Johnson JI, Kirsch JA, Reep RL, Switzer RC 3rd (1994) Phylogeny through brain traits: more characters for the analysis of mammalian evolution. Brain Behav Evol 43:319–347
Jones G, Teeling EC (2006) The evolution of echolocation in bats. Trends Ecol Evol 21:149–156
Kerr KM, Agster KL, Furtak SC, Burwell RD (2007) Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus 17:697–708
Kingdon J (1984) Bats: fruit bats. In Kingdon J (ed) East African mammals: an atlas of evolution in Africa: Part A Insectivores and bats, vol 2. The University of Chicago Press, Hampshire, pp 117–174
Klink R, Alonso A (1997a) Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus 7:571–583
Klink R, Alonso A (1997b) Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol 77:1813–1828
Kruska D (1975) Comparative quantitative study on brains of wild and laboratory rats. II. Comparison of size of allocortical brain centers. J Hirnforsch 16:485–496
Lapointe F, Baron G, Legendre P (1999) Encephalization, adaptation and evolution of chiroptera: a statistical analysis with further evidence for bat monophyly. Brain Behav Evol 54:119–126
Lorente de Nó R (1933) Studies on the structure of the cerebral cortex. J Psychol Neurol 45:381–438
Maseko BC, Manger PR (2007) Distribution and morphology of cholinergic, catecholaminergic and serotonergic neurons in the brain of Schreiber’s long-fingered bat, Miniopterus schreibersii. J Chem Neuroanat 34:80–94
Maseko BC, Bourne JA, Manger PR (2007) Distribution and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brain of the Egyptian rousette flying fox, Rousettus aegyptiacus. J Chem Neuroanat 34:108–127
Mikkonen M, Pitkänen A, Soininen H, Alafuzoff I, Miettinen R (2000) Morphology of spiny neurons in the human entorhinal cortex: intracellular filling with lucifer yellow. Neuroscience 96:515–522
Morrison JH, Lewis DA, Campbell MJ, Huntley GW, Benson DL, Bouras C (1987) A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer’s disease. Brain Res 416:331–336
Moser EI, Kropff E, Moser MB (2008) Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci 31:69–89
Mulders WH, West MJ, Slomianka L (1997) Neuron numbers in the presubiculum, parasubiculum, and entorhinal area of the rat. J Comp Neurol 385:83–94
O’Keefe J, Burgess N (2005) Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus 15:853–866
Paoletti P, Vergnano AM, Barbour B, Casado M (2009) Zinc at glutamatergic synapses. Neuroscience 158:126–136
Perez-Clausell J, Danscher G (1985) Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res 337:91–98
Peters A, Jones EG (1984) Classification of cortical neurons. Classification of cortical neurons. In: Jones EG, Peters A (eds) Cerebral Cortex, vol 1. Plenum Press, New York, pp 107–121
Pettigrew JD, Jamieson BG, Robson SK, Hall LS, McAnally KI, Cooper HM (1989) Phylogenetic relations between microbats, megabats and primates (Mammalia: Chiroptera and Primates). Philos Trans R Soc Lond B Biol Sci 325:489–559
Pettigrew JD, Maseko BC, Manger PR (2008) Primate-like retinotectal decussation in an echolocating megabat, Rousettus aegyptiacus. Neuroscience 153:226–231
Pitkänen A, Amaral DG (1993) Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: the hippocampal formation. J Comp Neurol 331:37–74
Ramón y Cajal S (1988) On a special ganglion of the spheno-occipital cortex. In: DeFelipe J, Jones EG (eds) Cajal on the cerebral cortex. An annoted translation of the complete writings. Oxford University Press, New York, pp 363–376
Rose M (1912) Histologische lokalisation der Grosshirnrinde bei Kleinen Saugetierein (Rodentia, Insectivora, Chiroptera). J Psychol Neurol 19:119–207
Rose M (1926) Der Allocortex bei Tier und Mensch. I. Teil. J Psychol Neurol 34:1–110
Rosene DL, Van Hoesen GW (1987) The hippocampal formation of the primate brain. A review of some comparative aspects of cytoarchitecture and connections. In: Jones EG, Peters A (eds) Cerebral cortex, vol 6. Plenum, New York, pp 345–456
Saleem KS, Price JL, Hashikawa T (2007) Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol 500:973–1006
Schwartz SP, Coleman PD (1981) Neurons of origin of the perforant path. Exp Neurol 74:305–312
Simmons NB, Seymour KL, Habersetzer J, Gunnell GF (2008) Primitive early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451:818–821
Slomianka L (1992) Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience 48:325–352
Slomianka L, Geneser FA (1991) Distribution of acetylcholinesterase in the hippocampal region of the mouse: I. Entorhinal area, parasubiculum, retrosplenial area, and presubiculum. J Comp Neurol 303:339–354
Slomianka L, West MJ (1989) Comparative quantitative study of the hippocampal region of two closely related species of wild mice: interspecific and intraspecific variations in volumes of hippocampal components. J Comp Neurol 280:544–552
Slomianka L, West MJ (2005) Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience 136:757–767
Stephan H, Baron G, Frahm HD, Stephan M (1986) Comparison of the size of brains and brain structures of mammals. Z Mikrosk Anat Forsch 100:189–212
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA 80:6126–6130
Suzuki W, Amaral DG (1994) Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci 14:1856–1877
Swanson LW, Cowan WM (1977) An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172:49–84
Swanson LW, Köhler C (1986) Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci 6:3010–3023
Tandrup T (1993) A method for unbiased and efficient estimation of number and mean volume of specified neuron subtypes in rat dorsal root ganglion. J Comp Neurol 329:269–276
Teeling EC, Scally M, Kao DJ, Romagnoli ML, Springer MS, Stanhope MJ (2000) Molecular evidence regarding the origin of echolocation and flight in bats. Nature 403:188–192
Tuñón T, Insausti R, Ferrer I, Sobreviela T, Soriano E (1992) Parvalbumin and calbindin D-28K in the human entorhinal cortex. An immunohistochemical study. Brain Res 589:24–32
Ulanovsky N, Moss CF (2007) Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci 10:224–233
Uva L, Grüschke S, Biella G, De Curtis M, Witter MP (2004) Cytoarchitectonic characterization of the parahippocampal region of the guinea pig. J Comp Neurol 474:289–303
van der Linden S, Lopes da Silva FH (1998) Comparison of the electrophysiology and morphology of layers III and II neurons of the rat medial entorhinal cortex in vitro. Eur J Neurosci 10:1479–1489
van Groen T (2001) Entorhinal cortex of the mouse: cytoarchitectonical organization. Hippocampus 11:397–407
van Groen T, Miettinen P, Kadish I (2003) The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus 13:133–149
van Hoesen G, Pandya DN (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res 95:1–24
West MJ, Slomianka L, Gundersen HJG (1991) Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Wouterlood FG, Hartig W, Bruckner G, Witter MP (1995) Parvalbumin-immunoreactive neurons in the entorhinal cortex of the rat: localization, morphology, connectivity and ultrastructure. J Neurocytol 24:135–153
Wóznicka A, Malinowska M, Kosmal A (2006) Cytoarchitectonic organization of the entorhinal cortex of the canine brain. Brain Res Rev 52:346–367
Acknowledgments
We thank Prof. Menno Witter for a critical reading of the manuscript. We are grateful for the help of Mr. Ben Agwanda (National Museums of Kenya, Nairobi), and Dr. Robert Kityo (Makerere University, Kampala) for guidance on the species biology and ecology and logistical support, Mr. Francis Muchemi (National Museums of Kenya, Nairobi), and Dr. Joseph M. Bukenya (Rubaga Hospital, Kampala) for assistance in the capture. Dr. Urs Ziegler (ZMB, Zürich) kindly introduced us to the 3D modelling software. This work was supported by grants from Rita Levi Montalcini Fellowship for African Women in Neuroscience, International Brain Research Organisation, National Centre for Competence in Research (NCCR) Neural Plasticity and Repair, and Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatome, C.W., Slomianka, L., Mwangi, D.K. et al. The entorhinal cortex of the Megachiroptera: a comparative study of Wahlberg’s epauletted fruit bat and the straw-coloured fruit bat. Brain Struct Funct 214, 375–393 (2010). https://doi.org/10.1007/s00429-010-0239-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-010-0239-z