Abstract
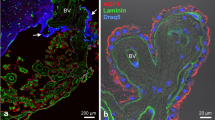
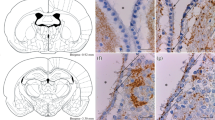
Aquaporins are selective water channel proteins critical in volume homeostasis. In the CNS AQP4 predominates, localized mainly in the glia limitans, the perivascular endfeet and ependyma. The present immunofluorescent study reveals the distribution of aquaporin-4 in the circumventricular organs in rat and chicken brains. The ventricular ependyma (especially in the third one), the subfornical organ, the area postrema, the rat pineal body (in part), and the vascular organ of lamina terminalis were marked by intense immunopositivity. Several areas, however, proved to be immunonegative: the central canal, the subcommissural organ, the ependymal zone of the median eminence in rat but its whole thickness in chicken, the subtrochlear organ, and the paraventricular organ. The immunostaining of the lateral septal and subseptal organs were similar to their environment. Results on developing rats suggested that the aquaporin-4 immunonegativity is a secondary phenomenon. Surveying other structural and functional features, no clear explanation of the heterogeneous occurrence of aquaporin-4 was found. The absence of aquaporin-4 seems to correlate with some features of the “ependymal organs” (thickened, pseudostratified ependyma, presence of blood–brain barrier) and with the avoidance of GFAP. On the other hand, the organs rich in aquaporin-4 have features of the “hypendymal organs” (glial and vascular plexus but no blood–brain barrier). There are organs, however, which do not fit into either group completely, i.e. the lateral septal and subseptal organs. Presence of tight junctions coincides with the absence of aquaporin-4 in the ependyma of spinal cord, the subcommissural organ and the ependyma of median eminence.











Similar content being viewed by others
Abbreviations
- AQP4:
-
Aquaporin-4
- CVO:
-
Circumventricular organ
- GFAP:
-
Glial fibrillary acidic protein
- LSO:
-
Lateral septal organ
- OVLT:
-
Vascular organ of lamina terminalis (organon vasculosum laminae terminalis)
- PVO:
-
Paraventricular organ
- SCO:
-
Subcommissural organ
- SFO:
-
Subfornical organ
- STO:
-
Subtrochlear organ
References
Agre P, Kozono D (2003) Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 555:72–78
Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S (2002) Aquaporin water channels from atomic structure to clinical medicine. J Physiol 542:3–16
Amiry-Moghaddam M, Ottersen OP (2003) The molecular basis of water transport in the brain. Nat Rev Neurosci 4:991–1001
Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug F-M, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A (2003) An α-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA 100:2106–2111
Badaut J, Petit JM, Brunet JF, Magistretti PJ, Charriaut-Marlangue C, Regli L (2004) Distribution of Aquaporin 9 in the adult rat brain: preferential expression in catecholaminergic neurons and in glial cells. Neuroscience 128:27–38
Bouchard P, Ravet V, Meiniel R, Creveaux I, Meiniel A, Vellet A, Vigues B (1999) Use of a heterologous monoclonal antibody for cloning and detection of glial fibrillary acidic protein in the bovine ventricular ependyma. Cell Tiss Res 298:207–216
Brightman MW, Reese T (1969) Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40:648–677
Chouaf L, Didier-Bazes M, Hardin H, Aguera M, Fevre-Montagne M, Voutsinos B, Belin MF (1991) Developmental expression of glial markers in ependymocytes of the rat subcommissural organ: role of the environment. Cell Tiss Res 266:553–561
D’Amelio FE, Gibbs MA, Mehler WR, Eng LF (1985) Immunocytochemical localization of glial fibrillary acidic protein in the area postrema of the cat: light and electron microscopic study. Brain Res 330:146–149
De Vitry F, Picart R, Jacque C, Tixier-Vidal (1981) Glial fibrillary acidic protein. A cellular marker of tanycytes in the mouse hypothalamus. Dev Neurosci 4(6):457–460
Dellmann HD (1985) Fine structural organization of the subfornical organ. A concise review. Brain Res Bull 15:71–78
Dermietzel R (1975) Junctions in the central nervous system of the cat. IV. Interendothelial junctions of cerebral blood vessels from selected areas of the brain. Cell Tiss Res 164:45–62
Dermietzel R, Meller K, Tetzlaff W, Waelsch M (1977) In vivo and in vitro formation of the junctional complex in choroid epithelium. A freeze-etching study. Cell Tiss Res 181:427–441
Didier M, Harandi M, Aguera M, Bancel B, Tardy M, Fages C, Calas A, Stagaard M, Mollgardet M, Belin MF (1986) Differential immunocytochemical staining for glial fibrillary acidic protein, S-100 protein and glutamine syntethase in the rat subcommissural organ, nonspecialized ventricular ependyma and adjacent neuropil. Cell Tiss Res 245:343–351
Elkjaer M-L, Vajda Z, Nejsum LN, Kwon T-H, Jensen UB, Amiry-Moghaddam M, Frøkiaer J, Nielsen S (2000) Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Comm 276:1118–1128
Engel A, Fujiyoshi Y, Agre P (2000) The importance of aquaporin water channel protein structures. J EMBO 19:800–806
Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D (1995) Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci 108:2993–3002
Furman C, Gorelick-Feldman D, Davidson K, Yasumura T, Neely J, Agre P, Rash J (2003) Aquaporin-4 square array assembly: Opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci USA 100(23):13609–13614
Gotow T, Hashimoto PH (1982) Intercellular junctions between specialized ependymal cells in the subcommissural organ of the rat. J Neurocytol 11:363–379
Gobron S, Creveaux I, Meiniel R, Didier R, Herbet A, Bamdad M, Bitar F, Dastugue B, Meiniel A (2000) Subcommissural organ/Reissner’s fiber complex: characterization of SCO-spondin, a glycoprotein with potent activity on neurite outgrowth. Glia 32:177–191
Gwyn DG, Wolstencroft JH (1968) Cholinesterases in the area subpostrema: a region adjacent to the area postrema in the cat. J Comp Neurol 133:289–308
Hajós F, Kálmán M (1989) Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. II. Mesencephalon, rhombencephalon and spinal cord. Exp Brain Res 78:164–173
Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS (1994) Molecular cloning of a mercurial- insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 269:5497–5500
Hatton JD, Ellisman MH (1982) The distribution of orthogonal arrays in the freeze-fractured rat eminence. J Neurocytol 11:335–349
Hatakeyama S, Yoshida Y, Tani T, Koyama Y, Nihei K, Ohshiro K, Kamiie JI, Yaoita E, Suda T, Hatakeyama K, Yamamoto T (2001) Cloning of a new aquaporin (AQP10) abundantly expressed in duodenum and jejunum. Biochem Biophys Res Comm 287:814–819
Hirunagi K, Rommel E, Korf HW (1995) Ultrastructure of cerebrospinal fluid-contacting neurons immunoreactive to vasoactive intestinal peptide and properties of the blood-brain barrier in the lateral septal organ of the duck. Cell Tiss Res 279:123–133
Hofer H (1959) Zur Morphologie der cirkumventrikulären Organe des Zwischenhirnes der Säugetiere. Verh Dtsch Zool Ges 22:202–251
Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P (1994) Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA 91:13052–13056
Kálmán M, Hajós F (1989) Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. I. Forebrain. Exp Brain Res 78:147–163
Kálmán M, Székely AD, Csillag A (1993) Distribution of glial fibrillary acidic protein-immunopositive structures in the brain of the domestic chicken (Gallus domesticus). J Comp Neurol 330:221–237
Kálmán M, Székely A, Csillag A (1998) Distribution of glial fibrillary acidic protein and vimentin immunopositive elements in the developing chicken brain from hatch to adulthood. Anat Embryol 198:213–235
Knigge KM, Scott DE (1970) Structure and function of the median eminence. Am J Anat 129:223–244
Korf HW, Fahrenkrug J (1984) Ependymal and neuronal specializations in the lateral ventricle of the Pekin duck, Anas platyrhynchos. Cell Tiss Res 236:217–227
Krisch B (1986) The functional and structural borders between the CSF- and blood-dominated milieus in the choroid plexuses and the area postrema of the rat. Cell Tiss Res 245:101–115
Krisch B, Leonhardt H, Buchheim W (1978a) The functional and structural border of the neurohemal region of the median eminence. Cell Tiss Res 192:327–339
Krisch B, Leonhardt H, Buchheim W (1978b) The functional and structural border between the CSF- and blood-milieu in the circumventricular organs (organum vasculosum laminae terminalis, subfornical organ, area postrema) of the rat. Cell Tiss Res 195:485–579
Kuenzel WJ, Blähser S (1994) Vasoactive intestinal polypeptide (VIP)-containing neurons: distribution throughout the brain of the chick (Gallus domesticus) with focus upon the lateral septal organ. Cell Tiss Res 275:91–107
Kuenzel WJ, Masson M (1988) A Stereotaxic Atlas of the Brain of the Chick (Gallus domesticus). The Johns Hopkins University Press, Baltimore
Kuenzel WJ, van Tienhoven A (1982) Nomenclature and location of avian hypothalamic nuclei and circumventricular organs. J Comp Neurol 206:293–313
Lechan RM (1996) Functional microanatomy of the hypophysial-pituitary axis. Front Horm Res 20:2–40
Legait H, Legait E (1958) Paraphyse et organa subfornical dans la série des vertébrés. CR Assoc Anat 99:427–435
Linser PJ (1985) Multiple marker analysis in the avian optic tectum reveals three classes of neuroglia and carbonic anhydrase-containing neurons. J Neurosci 5:2388–2396
Ludwin SK, Kosek JC, Eng LF (1976) The topographical distribution of S-100 and GFA proteins in the adult rat brain. An immunocytochemical study using horseradish peroxidase labelled antibodies. J Comp Neurol 165:197–208
Mack A, Neuhaus J, Wolburg H (1987) Relationship between orthogonal arrays of particles and tight junctions as demonstrated in cells of the ventricular wall of the rat brain. Cell Tiss Res 248:619–625
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Mestres P, Rascher K (1994) The ventricular system of the pigeon brain:a scanning electron microscope study. J Anat 184:35–58
Monroe BG, Holmes EM (1982) The freeze-fractured median eminence. I. Development of intracellular junctions in the ependyma of the 3rd ventricle of the rat. Cell Tiss Res 222:389–408
Mugnaini E (1986) Cell junctions of astrocytes, ependyma, and related cells in the mammalian central nervous system, with emphasis on the hypothesis of a generalized functional syncytium of supporting cells. In: Fedoroff S, Vernadakis A (eds) Astrocytes, vol 1. Academic Press, New York, pp 329–371
Møller M, Inglid A, Bock E (1978) Immunohistochemical demonstration of S-100 protein and GFA-protein in interstitial cells of rat pineal body. Brain Res 140:1–13
Murabe Y, Nishida K, Sano S (1981) Cells capable of uptake of horseradish peroxidase in some circumventricular organs of the rat. Cell Tiss Res 219:85–92
Nicchia GP, Frigeri A, Nico B, Ribatti D, Svelto M (2001) Tissue distribution and membrane localization of aquaporin-9 water channel: evidence for sex-linked differences in liver. J Histochem Cytochem 49:1547–1556
Nico B, Frigeri A, Nicchia GP, Quondamatteo F, Herken R, Errede M, Ribatti D, Svelto M, Roncali L (2001) Role of aquaporin-4 water channel in the development and integrity of the blood-brain barrier. J Cell Sci 114:1297–1307
Nico B, Frigeri A, Nicchia GP, Ribatti D, Quondamatteo F, Herken R, Girolamo F, Marzullo A, Svelto M, Roncali L (2003) Severe alternations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice. Glia 42:235–251
Nico B, Nicchia GP, Frigeri A, Corsi P, Mangieri D, Ribatti D, Svelto M, Roncali L (2004) Altered blood-brain barrier development in dystrophic mdx mice. Neuroscience 125:921–935
Nielsen S, Smith BL, Christiensen EI, Agre P (1993) Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci USA 90:7275–7279
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–180
Palkovits M (1986) Summary of structural and functional aspects of the circumventricular organs. In: Gross, PM (ed) Circumventricular organs and body fluids, vol. II. CRC Press, Boca Raton, pp 209–218
Papasozomenos SC (1983) Glial fibrillary acidic (GFA) protein-containing cells in the human pineal gland. J Neuropathol Exp Neurol 42:391–408
Petrov T, Howarth AG, Krukoff TL, Stevenson BR (1994) Distribution of the tight junction-associated protein ZO-1 in circumventricular organs of the CNS. Mol Brain Res 21:235–246
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114
Privat A (1977) The ependyma and subependymal layer of the young rat: A new contribution with freeze-fracture. Neuroscience 2:447–457
Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S (1998) Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA 95:11981–11986
Redecker P (1990) The glial architecture of the median eminence of the Mongolian gerbil (Meriones unguiculatus); a study of glial fibrillary acidic protein (GFAP) immunoreactivity in semithin sections. Acta Histochem 88:139–147
Reiner A, Perkel DJ, Bruce L, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild MJ, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED (2004) Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377–414
Rodriguez P, Bouchaud C (1996) The supra-ependymal innervation is not responsible for the repression of tight junctions in the rat cerebral ependyma. Neurobiology (Bp) 4:185–201
Roessmann U, Velasco ME, Sindely SD, Gambetti P (1980) Glial fibrillary acidic protein in ependymal cells during development. An immunocytochemical study. Brain Res 200:13–21
Röhlich P, Vígh B (1967) Electron microscopy of the paraventricular organ in the sparrow (Passer domesticus). Z Zellforsch Mikrosk Anat 80:229–245
Smith GM, Shine HD (1992) Immunofluorescent labeling of tight junctions in the rat brain and spinal cord. Int J Dev Neurosci 10:387–392
Takei Y, Tsuneki K, Kobayashi H (1978) Surface fine structure of the subfornical organ in the japanese quail, Coturnix coturnix japonica. Cell Tiss Res 191:389–404
Tsuneki K, Takei Y, Kobayashi H (1978) Parenchymal fine structure of the subfornical organ in the japanese quail, Coturnix coturnix japonica. Cell Tiss Res 191:405–419
Tsuneki K (1986) A survey of occurrence of about seventeen circumventricular organs in brains of various vertebrates with special reference to lower groups. J Hirnforsch 27:441–470
Uryu K, Hirunagi K, Fujioka T (1988) Specializated ependyma in the posterior mesencephalon of the chicken: the fine structure of the subtrochlear organ. Cell Tiss Res 254:531–538
Vajda Z, Pedersen M, Füchtbauer E-M, Wertz K, Stødkilde-Jørgensen H, Sulyok E, Dóczi T, Neely JD, Agre P, Frøkiaer J, Nielsen S (2002) Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci USA 99:13131–13136
Venero JL, Vizuete ML, Ilundain AA, Machado A, Echevarria M, Cano J (1999) Detailed localization of aquaporin-4 messenger RNA in the CNS: preferential expression in periventricular organs. Neuroscience 94:239–250
Venero JL, Vizuete ML, Machado A, Cano J (2001) Aquaporins in the central nervous system. Prog Neurobiol 63:321–336
Verbavatz JM, Ma T, Gobin R, Verkman AS (1997) Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci 110:2855–2860
Viehweg J, Naumann WW (1996) Radial secretory glia conserved in the postnatal vertebrate brain: a study in the rat. Anat Embryol (Berl) 194:355–363
Vígh B (1971) Das Paraventrikularorgan und das zirkumventrikuläre System des Gehirns. Stud Biol Hung 10:9–142
Vígh B, Manzano e Silva MJ, Frank CL, David C, Czirok SJ, Vincze C, Rácz G, Lukáts A, Szél Á (2004) The circumventricular organs of the brain: Do they represent a cerebrospinal fluid-dependent regulatory system? Med Hypotheses Res 1:77–100
Wells T (1998) Vesicular osmometers, vasopression secretion and aquaporin-4: a new mechanism for osmoreception? Mol Cell Endocrinol 136:103–107
Wolburg H (1995) Orthogonal arrays of intramembraneous particles: a review with special reference to astrocytes. J Hirnforsch 36:239–258
Yamaguchi K, Morimoto A, Murakami N (1993) Organum vasculosum laminae terminalis (OVLT) in rabbit and rat: topographic studies. J Comp Neurol 330:352–362
Yamamoto N, Sobue K, Miyachi T, Inagaki M, Mima Y, Kakuya H, Asai K (2001) Differential regulation of aquaporin expression in astrocytes by protein kinase C. Mol Brain Res 95:110–116
Yamamoto N, Sobue K, Fujita M, Katsuve H, Asai K (2002) Differential regulation of aquaporin-5 and aquaporin-9 expression in astrocytes by protein kinase A. Mol Brain Res 104:96–102
Yoneda K, Yamamoto N, Asai K, Sobue K, Fujita Y, Fujita M, Mase M, Yamada K, Nakanishi M, Tada T, Miura Y, Kato T (2001) Regulation of aquaporin-4 expression in astrocytes. Mol Brain Res 89:94–102
Zhuang X, Silverman AJ, Silver R (1996) Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol 31:393–403
Acknowledgements
This study was supported by the Department of Anatomy, Histology and Embryology and the PhD School of the Semmelweis University. The technical assistance of Ms. S. Deák and Ms. E. Oszwald is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goren, O., Adorján, I. & Kálmán, M. Heterogeneous occurrence of aquaporin-4 in the ependyma and in the circumventricular organs in rat and chicken. Anat Embryol 211, 155–172 (2006). https://doi.org/10.1007/s00429-005-0067-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-005-0067-8