Abstract
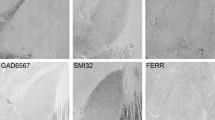
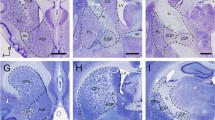
This study provides a light-microscopic description of the organization, morphology and number of neurons in the subthalamic nucleus (STN) of the Göttingen minipig. It is based on histological material stained with Nissl, Golgi and autometallographic techniques, and employs design-based stereological estimation of the total neuron number. The organization of several neurotransmitters in the STN has been evaluated in histological preparations stained for acetylcholinesterase (AChE) and immunostained for choline acetyltransferase (ChAT), tyrosine hydroxylase (TH), glutamic acid decarboxylase (GAD) and glutamate. In all of the stained preparations the STN appeared as a distinct lens-shaped structure located in the caudal diencephalon, medial to the internal capsule and ventrolateral to the zona incerta. Rostrally, the STN approached the globus pallidus pars interna, whereas caudally the ventromedial part of the STN was adjacent to the rostral part of the substantia nigra pars compacta (SNc), where some of the neurons of the two nuclei merged. The neurons in the STN had medium-sized (25–40 μm) ovoid or fusiform cell bodies, from which three to six large dendrites emanated in a direction predominantly parallel to the long axis of the STN. Immunohistochemistry revealed that most of the subthalamic neurons were glutamatergic and differed significantly in appearance from the large stellate TH-positive cells of the adjacent SNc. Numerous TH-positive bouton-rich fibers traversed the STN. The GAD-staining revealed a large number of terminals within the boundaries of the STN. The STN was highly AChE-positive, reflecting a prominent innervation by ChAT-positive terminals. The total number of subthalamic neurons in one hemisphere was estimated to be approximately 56,000. We conclude that the neuroarchitecture of the porcine STN is similar to primates, including humans, and appears well-suited for further studies examining the role of the STN in movement disorders.



Similar content being viewed by others
Abbreviations
- AChE:
-
Acetylcholinesterase
- AMG:
-
Autometallography
- asf:
-
Area sampling fraction
- ChAT:
-
Choline acetyltransferase
- CI:
-
Internal capsule
- DBS:
-
Deep brain stimulation
- fl:
-
Lenticular fasciculus
- GAD:
-
Glutamic acid decarboxylase
- GPe:
-
Globus pallidus pars externa
- GPi:
-
Globus pallidus pars interna
- mfb:
-
Medial forebrain bundle
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- nsp:
-
Nigrostriatal pathway
- SN:
-
Substantia nigra
- SNc:
-
Substantia nigra, pars compacta
- SNl:
-
Substantia nigra, pars lateralis
- ssf:
-
Section sampling fraction
- STN:
-
Subthalamic nucleus
- TBS:
-
Tris-buffered saline
- TH:
-
Tyrosine hydroxylase
- VTA:
-
Ventral tegmental area
- ZI:
-
Zona incerta
References
Afsharpour S (1985) Light microscopic analysis of Golgi-impregnated rat subthalamic neurons. J Comp Neurol 236:1–13
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Alexander GE (1994) Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol 11:420–431
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Benabid AL, Benazzous A, Pollak P (2002) Mechanisms of deep brain stimulation. Mov Disord 17 Suppl 3:S73–S74
Benazzouz A, Hallett M (2000) Mechanism of action of deep brain stimulation. Neurology 55:S13–S16
Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B (1993) Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci 5:382–389
Benazzouz A, Boraud T, Feger J, Burbaud P, Bioulac B, Gross C (1996) Alleviation of experimental hemiparkinsonism by high-frequency stimulation of the subthalamic nucleus in primates: a comparison with L-Dopa treatment. Mov Disord 11:627–632
Bergman H, Wichmann T, DeLong MR (1990) Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249:1436–1438
Bergman H, Wichmann T, Karmon B, DeLong MR (1994) The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72:507–520
Bevan MD, Bolam JP (1995) Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci 15:7105–7120
Bevan MD, Francis CM, Bolam JP (1995) The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol 361:491–511
Bjarkam CR, Sørensen JC, Geneser FA (1997) Distribution and morphology of serotonin-immunoreactive neurons in the brainstem of the New Zealand white rabbit. J Comp Neurol 380:507–519
Bjarkam CR, Sørensen JC, Sunde NA, Geneser FA, Østergaard K (2001A) New strategies for the treatment of Parkinson’s disease hold considerable promise for the future management of neurodegenerative disorders. Biogerontology 2:193–207
Bjarkam CR, Pedersen M, Sørensen JC (2001B) New strategies for embedding, orientation and sectioning of small brain specimens enable direct correlation to MR-images, brain atlases, or use of unbiased stereology. J Neurosci Methods 108:153–159
Bjarkam CR, Andersen F, Larsen M, Watanabe H, Röhl L, Vafaee M, Cumming P, Gjedde A, Sørensen JC (2002) Subthalamic high frequency deep brain stimulation increases oxygen consumption in the cerebral cortex pointing towards increased thalamocortical motor activation. FENS Abstr vol 1, A091.2
Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy: Classical transmitters in the CNS. Elsevier, Amsterdam, pp 55–122
Boraud T, Bezard E, Bioulac B, Gross C (1996) High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett 215:17–20
Campbell GA, Eckardt MJ, Weight FF (1985) Dopaminergic mechanisms in subthalamic nucleus of the rat: analysis using horseradish peroxidase and microiontophoresis. Brain Res 333:261–270
Cumming P, Danielsen EH, Vafaee M, Falborg L, Steffensen E, Sørensen JC, Gillings N, Bender D, Marthi K, Andersen F, Munk O, Smith D, Møller A, Gjedde A (2001) Normalization of markers for dopamine innervation in striatum of MPTP-lesioned miniature pigs with intrastriatal grafts. Acta Neurol Scand 103:309–315
Dall AM, Danielsen EH, Sørensen JC, Andersen F, Møller A, Zimmer J, Gjedde AH, Cumming P (2002) Quantitative [18F] fluorodopa/PET and histology of fetal mesencephalic dopaminergic grafts to the striatum of MPTP-poisoned minipigs. Cell Transplant 11:733–746
Danielsen EH, Cumming P, Andersen F, Bender D, Brevig T, Falborg L, Gee A, Gillings NM, et al. (2000) The DaNeX study of embryonic mesencephalic, dopaminergic tissue grafted to a minipig model of Parkinson’s disease: preliminary findings of effect of MPTP poisoning on striatal dopaminergic markers. Cell Transplant 9:247–259
Danscher G (1996) The autometallographic zinc-sulphide method. A new approach involving in vivo creation of nanometer-sized zinc sulphide crystal lattices in zinc-enriched synaptic and secretory vesicles. Histochem J 28:361–373
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–285
Dostrovsky JO, Lozano AM (2002) Mechanisms of deep brain stimulation. Mov Disord 17 Suppl 3:S63–S68
Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP (1999) Stereotaxic atlas of the pig brain. Brain Res Bull 49:1–137
Gao DM, Benazzouz A, Piallat B, Bressand K, Ilinsky IA, Kultas-Ilinsky K, Benabid AL (1999) High-frequency stimulation of the subthalamic nucleus suppresses experimental resting tremor in the monkey. Neuroscience 88:201–212
Geneser-Jensen FA, Blackstad TW (1971) Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat 114:460–481
Goodman S, Check E (2002) The great primate debate. Nature 417:684–687
Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM (2002) Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol 445:238–255
Hassani OK, Francois C, Yelnik J, Feger J (1997) Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Res 749:88–94
Hedreen JC (1999) Tyrosine hydroxylase-immunoreactive elements in the human globus pallidus and subthalamic nucleus. J Comp Neurol 409:400–410
Iwahori N (1978) A Golgi study on the subthalamic nucleus of the cat. J Comp Neurol 182:383–397
Just H, Østergaard K (2002) Health-related quality of life in patients with advanced Parkinson’s disease treated with deep brain stimulation of the subthalamic nuclei. Mov Disord 17:539–545
Kaufmann DL, Houser CR, Tobin AJ (1991) Two forms of the γ-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem 56:720–723
Kita H, Chang HT, Kitai ST (1983) The morphology of intracellularly labeled rat subthalamic neurons: a light microscopic analysis. J Comp Neurol 215:245–257
Kitai ST, Kita H (1987) Anatomy and physiology of the subthalamic nucleus: A driving force of the basal ganglia. In: Carpenter MB, Jayaraman A (eds) The Basal Ganglia II—Structure and Function: Current Concepts, Advances in Behavioral Biology. Plenum, New York, pp 357–373
Kodama S (1928) Beiträge zur normalen Anatomie des Corpus Luysi beim Menschen. Arbeiten an den Anatomischen Institute, Sendei 13:221–254
Krack P, Pollak P, Limousin P, Hoffmann D, Benazzouz A, Le Bas JF, Koudsie A, Benabid AL (1998A) Opposite motor effects of pallidal stimulation in Parkinson’s disease. Ann Neurol 43:180–192
Krack P, Pollak P, Limousin P, Hoffmann D, Xie J, Benazzouz A, Benabid AL (1998B) Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain 121:451–457
Krack P, Poepping M, Weinert D, Schrader B, Deuschl G (2000) Thalamic, pallidal, or subthalamic surgery for Parkinson’s disease? J Neurol 247 Suppl 2:122–134
Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, Lang AE (1998) Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology 51:850–855
Larsen M, Bjarkam CR, Stoltenberg M, Sørensen JC, Danscher G (2003) An autometallographic technique for myelin staining in formaldehyde-fixed tissue. Histol Histopathol 18:1125–1130
Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL (1998) Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 339:1105–1111
Mesulam MM, Mash D, Hersh L, Bothwell M, Geula C (1992) Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J Comp Neurol 323:252–268
Mikkelsen M, Møller A, Jensen LH, Pedersen A, Harajehi JB, Pakkenberg H (1999) MPTP-induced Parkinsonism in minipigs: A behavioral, biochemical, and histological study. Neurotoxicol Teratol 21:169–175
Montgomery EB Jr, Baker KB (2000) Mechanisms of deep brain stimulation and future technical developments. Neurol Res 22:259–266
Oorschot DE (1996) Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the Cavalieri and optical disector methods. J Comp Neurol 366:580–599
Overton PG, O’Callaghan JF, Greenfield SA (1995) Possible intermixing of neurons from the subthalamic nucleus and substantia nigra pars compacta in the guinea-pig. Exp Brain Res 107:151–165
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev 20:128–154
Piallat B, Benazzouz A, Benabid AL (1996) Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci 8:1408–1414
Piallat B, Benazzouz A, Benabid AL (1999) Neuroprotective effect of chronic inactivation of the subthalamic nucleus in a rat model of Parkinson’s disease. J Neural Transm Suppl 55:71–77
Prensa L, Cossette M, Parent A (2000) Dopaminergic innervation of human basal ganglia. J Chem Neuroanat 20:207–213
Rafols JA, Fox CA (1976) The neurons in the primate subthalamic nucleus: a Golgi and electron microscopic study. J Comp Neurol 168:75–111
Rodriguez MC, Obeso JA, Olanow CW (1998) Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol 44:S175–S188
Rodriguez-Oroz MC, Gorospe A, Guridi J, Ramos E, Linazasoro G, Rodriguez-Palmero M, Obeso JA (2000) Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. Neurology 55:S45–S51
Romeis B (1989) Mikroskopische Technik. Urban & Schwarzenberg, München
Smith Y, Parent A (1988) Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res 453:353–356
Sørensen JC, Bjarkam CR, Danielsen EH, Simonsen CZ, Geneser FA (2000) Oriented sectioning of irregular tissue blocks in relation to computerized scanning modalities: results from the domestic pig brain. J Neurosci Methods 104:93–98
Tronnier VM, Krause M, Heck A, Kronenbürger M, Bonsanto MM, Tronnier J, Fogel W (1999) Deep brain stimulation for the treatment of movement disorders. Neurology, Psychiatry and Brain Research 6:199–212
Vitek JL (2002) Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 17 Suppl 3:S69–S72
Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V (2001) Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology 56:548–551
Watanabe H, Andersen F, Simonsen CZ, Evans SM, Gjedde A, Cumming P (2001) MR-based statistical atlas of the Göttingen minipig brain. Neuroimage 14:1089–1096
West MJ (1999) Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci 22:51–61
West MJ, Gundersen HJ (1990) Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol 296:1–22
West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Whittier JR, Mettler FA (1949) Studies on the subthalamus of the rhesus monkey. J Comp Neurol 90:281–317
Wichmann T, Bergman H, DeLong MR (1994) The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 72:521–530
Yelnik J, Percheron G (1979) Subthalamic neurons in primates: a quantitative and comparative analysis. Neuroscience 4:1717–1743
Østergaard K (1993) Organotypic slice cultures of the rat striatum – I. A histochemical and immunocytochemical study of acetylcholinesterase, choline acetyltransferase, glutamate decarboxylase and GABA. Neuroscience 53:679–693
Østergaard K, Holm IE, Zimmer J (1992) Tyrosine hydroxylase and acetylcholinesterase in the domestic pig mesencephalon: an immunocytochemical and histochemical study. J Comp Neurol 322:149–166
Østergaard K, Sunde NA, Dupont E (2002) Effects of bilateral stimulation of the subthalamic nucleus in patients with severe Parkinson’s disease and motor fluctuations. Mov Disord 17:693–700
Acknowledgments
The authors acknowledge with gratitude the skillful assistance of Ms. D. Jensen, Ms. L. Munkøe, Ms. K. Wiedemann, Mr. A. Meier, Mr. T. Nielsen, and Ms. H. Mikkelsen. We acknowledge the valuable discussions with Dr. M. Stoltenberg, Dr. M.S. Jensen, Dr. G. Danscher, and Dr. F.A. Geneser.
The following supported the study: the Danish Parkinson Association, the Petrus Andersen Foundation, the Foundation of Politimester J.P.N. Colind og Hustru Asmine Colind, the Foundation of Etatsraad C.G. Filtenborg og Hustru Marie Filtenborg, the Helga og Peter Korning Foundation, the Aarhus University Research Foundation, the Alice Brenaa Foundation, the Danish Medical Research Council, the Foundation for the Advancement of Medical Science, the Lily Benthine Lund Foundation, the Medical Society of Aarhus County, the Novo Nordic Foundation, the Aase and Ejnar Danielsen Foundation, the Foundation of 2/7 1984 against Parkinson’s Disease, the Foundation of Direktør Ib Henriksen, and the Foundation of King Christian the Tenth.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larsen, M., Bjarkam, C.R., Østergaard, K. et al. The anatomy of the porcine subthalamic nucleus evaluated with immunohistochemistry and design-based stereology. Anat Embryol 208, 239–247 (2004). https://doi.org/10.1007/s00429-004-0395-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-004-0395-0