Abstract
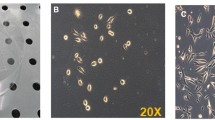
The regeneration of adult skeletal muscle is mediated by satellite cells. Classically, these are considered to be somitically derived cells that colonize the limbs during early embryogenesis. The striated urethral sphincter presents specific developmental characteristics that distinguish it from skeletal muscles, such as the non-somitic origin of its precursor cells and the late formation of its myofibers. This prompted us to determine whether the striated urethral sphincter can regenerate after injury by the same mechanism as skeletal muscles. By means of the single myofiber explant culture technique we investigated the presence of satellite cells in the striated urethral sphincter of male mice and evaluated their ability to recapitulate a myogenic program. In addition, a myotoxic substance (notexin) was injected into the sphincter in order to provoke rapid destruction of the myofibers; the regeneration process was studied by means of electrophysiological and histological techniques. Satellite cells expressing pax7 were found attached to the sphincteric myofibers. They proliferated and expressed MyoD, Myf5 and desmin after 2 days in culture. After 10 days, they formed multinucleated myotubes expressing α-actinin-2. In vivo, complete recovery of the striated urethral sphincter, as assessed by normalization of muscle strength and of myofiber number and diameter, was observed after 3 weeks, and resulted from the fusion of myogenic cells. These results demonstrate that the striated urethral sphincter can regenerate by means of a myogenic program involving intrinsic satellite cells. The therapeutic implications of this knowledge and the possible origin of the sphincteric satellite cells are discussed.




Similar content being viewed by others
References
Alameddine HS, Louboutin JP, Dehaupas M, Sebille A, Fardeau M (1994) Functional recovery induced by satellite cell grafts in irreversibly injured muscles. Cell Transplant 3:3-14
Allen RE, Temm-Grove CJ, Sheehan SM, Rice G (1997) Skeletal muscle satellite cells cultures. Methods Cell Biol 52:155–76
Ballanger P, Rischmann P. Female urinary incontinence (1999) An overview of a report presented to the French Urological Association. Eur Urol 36:165–174
Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1134
Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM (1992) Cloning and characterization of two human skeletal muscle alpha-actinin geneslocated on chromosomes 1 and 11. J Biol Chem 267:9281–9288
Bischoff R (1990) Interaction between satellite cells and skeletal muscle fibers. Development 109:943–952
Borirakchanyavat S, Baskin LS, Kogan BA Cunha GR (1997) Smooth and striated muscle development in the intrinsic urethral sphincter. J Urol 158:1119–1122
Bourdelat D, Barbet JP, Butler-Browne GS (1991) Fetal development of the urethral sphincter. Eur J Pediat Surg 2:35–38
Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS (1999) In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci 112:2895–2901
Dimpfl T, Jaeger C, Mueller-Felber W, Anthuber C, Hirsch A, Brandmaier R, Schuessler B (1998) Myogenic changes of the levator ani muscle in premenopausal women: the impact of vaginal delivery and age. Neurourol Urodyn 17:197–205
Dixon RW, Harris JB (1996) Myotoxic activity of the toxic phospholipase, notexin, from the venom of the Australian tiger snake. J Neuropath Exp Neur 55:1230–1237
Hale DS, Benson JT, Brubaker L, Heidkamp MC, Russell B (1999) Histologic analysis of needle biopsy of urethral sphincter from women with normal and stress incontinence with comparison of electromyographic findings. Am J Obstet Gynecol 180:342–348
Kablar B, Tajbakhsh S, Rudnicki MA (2000) Transdifferentiation of esophageal smooth to skeletal muscle is myogenic bHLH factor-dependent. Development 127:1627–1639
Lefaucheur JP, Sebille A (1995) The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscular Disord 5:501–509
Link BA, Nishi R (1998) Development of the avian iris and ciliary body: mechanisms of cellular differentiation during the smooth-to-striated muscle transition. Dev Biol 203:163–176
Parazzini F, Colli E, Origgi G, Surace M, Bianchi M, Benzi G, Artibani W (2000) Risk factors for urinary incontinence in women. Eur Urol 37:637–643
Partridge T, Morgan JE, Coulton Gr, Hoffmam EP, Kunnel LM (1989) Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal muscle precursor cells. Nature 337:176–179
Patapoutian A, Wold BJ, Wagner RA (1995) Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science 270:1818–1821
Perry RL, Rudnicki MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5: D750–767
Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA (1995) Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 31:773–779
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Sharp NJ, Kornegay JN, Bartlett RJ, Hung WY, Dykstra MJ (1993) Notexin-induced muscle injury in the dog. J Neurol Sci 116:73–81
Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB (1993) A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somatic from embryonic, fetal and newborn mouse myogenic cells. Development 117:1125–1133
Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, R Roy R, Edgerton VR (2002) Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 157:571–577
Volpe P, Biral D, Pizzo P, Salviati G, Margreth A (1993) Ontogenesis of chick iris intrinsic muscles: evidence for a smooth-to-striated muscle transition. Dev Biol 159:441–449
Ulmsten U, Ekman G, Giertz G, Malmstrom A (1987) Different biochemical composition of connective tissue in continent and stress incontinent women. Acta Obstet Gynecol Scand 66, 455–457
Yablonka-Reuveni Z, Rivera AJ (1994) Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol 164:588–603
Acknowledgments
We would like to thank Geoffrey Liu, Federica Montanaro and François Rivier for their critical reading of the manuscript and Saadia Eddahibi for her technical assistance. This work was supported by the Lavoisier foundation, the Association pour la Recherche contre le Cancer and the Association Française contre les Myopathies. The Muscular Dystrophy Foundation, Joshua Frase Foundation and the Crown Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yiou, R., Lefaucheur, JP. & Atala, A. The regeneration process of the striated urethral sphincter involves activation of intrinsic satellite cells. Anat Embryol 206, 429–435 (2003). https://doi.org/10.1007/s00429-003-0313-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-003-0313-x