Abstract
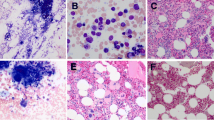
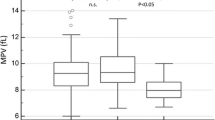
BCR–ABL-fusion-negative myeloproliferative neoplasms (MPNs) with myelofibrosis (MF) include primary MF, post-polycythemia vera MF and post-essential thrombocythemia MF. Clonal extramedullary hematopoiesis (EMH) can occur during MPN pathogenesis. Although histopathological bone-marrow (BM) features during clonal EMH have been investigated, those of the spleen have been poorly described. We analyzed splenectomy samples from 28 patients with MF and BM samples from 20 of them. Slides were stained with hematoxylin and eosin, reticulin, and trichrome, with immunohistochemical labeling of glycophorin A, myeloperoxidase, CD61, CD34, and CD117. We also subjected splenectomy and BM samples from six patients and spleen samples from seven patients to next-generation sequencing (NGS). Megakaryocyte-rich spleen nodules (MRSNs), seen in seven of the 28 patients, were significantly associated with megakaryocyte proliferation in the spleen (p = 0.04). We devised a grading system for spleen fibrosis (SF) and found that SF was increased in 20 of 28 patients. Notably, patients with SF were more likely to have MRSNs, suggesting that megakaryocytes might participate in SF, as previously described in BM. Comparisons of spleen and BM NGS findings of six patients’ specimens revealed identical mutational status in the two organs for half of the patients. We observed additional mutations in the spleen of two patients. However, the meaning of this finding remains unknown since there was a long interval between BM and spleen samplings (68 and 82 months, respectively).


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary information file).
Code availability
Not applicable.
References
James C, Ugo V, Le Couédic J-P, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu S, Casadevall N, Vainchenker W (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemiavera. Nature 434:1144–1148. https://doi.org/10.1038/nature03546
Levine RL, Wadleigh M, Cools J, Ebert B, Wernig G, Huntly BJP, Boggon TJ, Wlodarska I, Clark J, Moore S, Adelsperger J, Koo S, Lee J, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa R, Tefferi A, Griffin J, Eck M, Sellers W, Meyerson M, Golub T, Lee S, Gilliland D (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7:387–397. https://doi.org/10.1016/j.ccr.2005.03.023
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou G, Bench A, Boyd E, Curtin N, Scott M, Erber W, Green A (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061. https://doi.org/10.1016/S0140-6736(05)71142-9
Kralovics R, Passamonti F, Buser AS, Teo S, Tiedt R, Passweg J, Tichelli A, Cazzola M, Skoda R (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352:1779–1790. https://doi.org/10.1056/NEJMoa051113
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Azezov E, Li J, Kollmann K, Kent DG, Aziz A, Godfrey AL, Hinton J, Martincoren I, Van Loo P, Jones AV, Guglielmelli P, Tarpey P, Harding HP, Fitzpatrick JD, Goudie CT, Ortmann CA, Loughran SJ, Raine K, Jones DR, Butler AP, Teague JW, O’Meara S, McLaren S, Bianchi M, Silver Y, Dimitropoulou D, Bloxhan D, Mudie L, Maddison M, Robinson B, Keohane C, Maclean C, Hill K, Orchard K, Tauro S, Du M-Q, Greaves M, Bowen D, Huntly BJP, Harrison CN, Cross NCP, Ron D, Vannucchi AM, Papaemmanuil E, Campbell PJ, Green AR (2013) Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 369:2391–2405. https://doi.org/10.1056/NEJMoa1312542
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NCC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Carola CasettiSant’Antonio IE, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schönegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R (2013) Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 369:2379–2390. https://doi.org/10.1056/NEJMoa1311347
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert B, Gozo M, Cuker A, Wernig G, Moore S, Galinsky I, DeAngelo DJ, Clark J, Lee S, Golub T, Wadleigh M, Gilliland D, Levine R (2006) MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 3:e270. https://doi.org/10.1371/journal.pmed.0030270
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, Steensma DP, Elliott MA, Wolanskyj AP, Hogan WJ, McClure RF, Litzow MR, Gilliland DG, Tefferi A (2006) MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 108:3472–3476. https://doi.org/10.1182/blood-2006-04-018879
Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, Girsberger S, Lehmann T, Passweg J, Stern M, Beisel C, Kralovics R, Skoda RC (2014) Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 123:2220–2228. https://doi.org/10.1182/blood-2013-11-537167
Tefferi A, Nicolosi M, Mudireddy M, Szuber N, Finke CM, Lasho TL, Hanson CA, Ketterling RP, Pardanani A, Gangat N, Mannarelli C, Fanelli T, Guglielmelli P, Vannucchi A (2018) Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am J Hematol 93:348–355. https://doi.org/10.1002/ajh.24978
Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Massé A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, Lécluse Y, Plo I, Dreyfus F, Marzac C, Casadevall N, Lacombe C, Romana SP, Dessen P, Soulier J, Viguié F, Fontenay M, Vainchenker V, Bernard O (2009) Mutation in TET2 in myeloid cancers. N Engl J Med 360:2289–2301
Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W, Bruno E, Barosi G, Xu M, Hoffman R (2007) Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 110:986–993. https://doi.org/10.1182/blood-2006-12-064626
Mesa RA (2009) How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood 113:5394–5400. https://doi.org/10.1182/blood-2009-02-195974
Lataillade J-J, Pierre-Louis O, Hasselbalch HC, Uzan G, Jasmin C, Martyré MC, Le Bousse-Kerdilès MC (2008) Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood 112:3026–3035. https://doi.org/10.1182/blood-2008-06-158386
Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R (2010) The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res 70:3402–3410. https://doi.org/10.1158/0008-5472.CAN-09-3977
O’Malley DP, Kim YS, Perkins SL, Baldridge L, Juliar BE, Orazi A (2005) Morphologic and immunohistochemical evaluation of splenic hematopoietic proliferations in neoplastic and benign disorders. Mod Pathol 18:1550–1561. https://doi.org/10.1038/modpathol.3800480
Konoplev S, Hsieh P-P, Chang C-C, Medeiros L, Lin P (2007) Janus kinase 2 V617F mutation is detectable in spleen of patients with chronic myeloproliferative diseases suggesting a malignant nature of splenic extramedullary hematopoiesis. Hum Pathol 38:1760–1763. https://doi.org/10.1016/j.humpath.2007.04.004
Hsieh P-P, Olsen RJ, O’Malley DP, Konoplev S, Hussong J, Dunphy C, Perkins SL, Cheng L, Lin P, Chang C (2007) The role of Janus kinase 2 V617F mutation in extramedullary hematopoiesis of the spleen in neoplastic myeloid disorders. Mod Pathol 20:929–935. https://doi.org/10.1038/modpathol.3800826
Mesa RA (2001) Clinical correlates of splenic histopathology and splenic karyotype in myelofibrosis with myeloid metaplasia. Blood 97:3665–3667. https://doi.org/10.1182/blood.V97.11.3665
Prakash S, Hoffman R, Barouk S, Wang Y, Knowles D, Orazi A (2012) Splenic extramedullary hematopoietic proliferation in Philadelphia chromosome-negative myeloproliferative neoplasms: heterogeneous morphology and cytological composition. Mod Pathol 25:815–827. https://doi.org/10.1038/modpathol.2012.33
Zimran E, Tripodi J, Rampal R, Rappoport F, Zirkiev S, Hoffman R, Najfeld V (2018) Genomic characterization of spleens in patients with myelofibrosis. Haematologica 103:e446–e449. https://doi.org/10.3324/haematol.2018.193763
World Health Organization (WHO) Classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. Available at: https://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=24002. Accessed 11 May 2020
Larsen TS, Christensen JH, Hasselbalch HC, Pallisgaard N (2007) The JAK2 V617F mutation involves B- and T-lymphocyte lineages in a subgroup of patients with Philadelphia-chromosome negative chronic myeloproliferative disorders. Br J Haematol 136:745–751. https://doi.org/10.1111/j.1365-2141.2007.06497.x
Mansier O, Migeon M, Saint-Lézer A, James C, Verger E, Robin M, Socié G, Bidet A, Mahon FX, Cassinat B, Lippert E (2016) Quantification of the mutant CALR allelic burden by digital PCR. J Mol Diagn 18:68–74. https://doi.org/10.1016/j.jmoldx.2015.07.007
Boyd EM, Bench AJ, Goday-Fernández A, Anand S, Vaghela K, Beer P, Scott M, Bareford D, Green AR, Huntly B, Erber W (2010) Clinical utility of routine MPL exon 10 analysis in the diagnosis of essential thrombocythaemia and primary myelofibrosis. Br J Haematol 149:250–257. https://doi.org/10.1111/j.1365-2141.2010.08083.x
Brecqueville M, Rey J, Bertucci F, Coppin E, Finetti P, Carbuccia N, Cervera N, Gelsi-Boyer V, Arnoulet C, Gisserot O, Verrot D, Slama B, Vey N, Mozziconacci M-J, Birnbaum D, Murati A (2012) Mutation analysis of ASXL1, CBL, DNMT3A, IDH1, IDH2, JAK2, MPL, NF1, SF3B1, SUZ12, and TET2 in myeloproliferative neoplasms. Genes Chromosomes Cancer 51:743–755. https://doi.org/10.1002/gcc.21960
Porcu P, Neiman R, Orazi A (1998) Splenectomy in agnogenic myeloid metaplasia. Blood 93:2132–2134
Vainchenker W, Kralovics R (2017) Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 129:667–679. https://doi.org/10.1182/blood-2016-10-695940
Wang X, Prakash S, Lu M, Tripdo J, Ye F, Najfeld V, Li Y, Schwartz M, Weinberg R, Roda P, Orazi A, Hoffman R (2012) Spleens of myelofibrosis patients contain malignant hematopoietic stem cells. J Clin Invest 122:3888–3899. https://doi.org/10.1172/JCI64397
Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C, Nicolosi M, Pacilli A, Pardanani A, Rumi E, Rosti V, Hanson C, Mannelli F, Ketterling RP, Gangat N, Rambaldi A, Passamonti F, Barosi G, Barbui T, Cazzola M, Vannucchi AM, Tefferi A (2018) MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol 36:310–318. https://doi.org/10.1200/JCO.2017.76.4886
Grinfeld J, Nangalia J, Baxter EJ, Wedge DC, Angelopoulos N, Cantrill R, Godfrey AL, Papaemmanuil E, Gundem G, MacLean C, Cook J, O’Neil L, O’Meara S, Teague JW, Butler AP, Massie CE, Williams N, Nice FL, Andersen CL, Hasselbalch HC, Guglielmelli P, McMullin MF, Vannucchi AM, Harrison CN, Gerstung M, Green AR, Campbell PJ (2018) Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med 379:1416–1430. https://doi.org/10.1056/NEJMoa1716614
Acknowledgements
The authors thank the technical team of the Pathology Department, Haut-Lévêque Hospital, University Hospital of Bordeaux, for sectioning tissue samples and immunohistochemical labeling. The authors thank Janet Jacobson for editorial assistance and helpful discussions.
Author information
Authors and Affiliations
Contributions
A. Guy, M. Parrens, and J.-F. Viallard designed the research. A. Guy, A. Bidet, C. Ling, C. Caumont, L. Boureau, and M. Parrens carried out the research. A. Guy, A. Bidet, C. Ling, C. Caumont, L. Boureau, M. Parrens, and J.-F. Viallard analyzed data and wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of the University Hospital of Bordeaux (GP-CE2020-37).
Consent to participate
All patients consented to the use of their specimens for research.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jean-François Viallard and Marie Parrens are joint last authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guy, A., Bidet, A., Ling, C. et al. Novel findings of splenic extramedullary hematopoiesis during primary myelofibrosis, post-essential thrombocythemia, and post-polycythemia vera myelofibrosis . Virchows Arch 479, 755–764 (2021). https://doi.org/10.1007/s00428-021-03110-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03110-9