Abstract
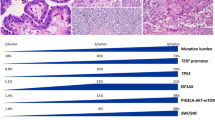
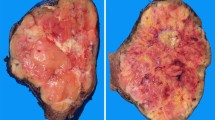
Anaplastic carcinoma (AC) is a rare but highly aggressive form of thyroid cancer. It mostly arises on a background of pre-existing well-differentiated cancer (WDC); however, whether it evolves directly from a WDC or originates as a second independent neoplasm is still to be defined. To obtain further insights into these mechanisms, we performed morphological, immunohistochemical, and next-generation sequencing analyses to compare AC and its associated WDC in a subset of 13 surgically resected specimens. Histologically, most WDC were of aggressive subtypes. Papillary carcinomas (8 cases; 62%) were tall cell (4/8), columnar (1/8), classic with hobnail features (1/8), classic and follicular variant in the remaining 2 cases; Hürthle cell and follicular carcinomas were present in 5 (38%) and in 1 (8%) patient, respectively. One patient harbored both a PTC, follicular variant, and a Hürthle cell carcinoma. We did not find any correlation between a histotype of WDC and a specific anaplastic growth pattern. Immunohistochemically, ACs retained pankeratin/PAX8 expression but with significantly lower levels than WDCs, and they tended to lose TTF1 expression, as can be expected within a dedifferentiation process. In addition, AC showed a more frequent expression of p63 and/or SMA, a mutated pattern of p53, and an abnormal expression of p16. Genetic analysis showed that the number of mutations was higher in AC than in the associated WDC, confirming a role of the progressive accumulation of genetic damage in this transition. We observed that mutations found in the WDCs were consistently identified in the anaplastic counterparts, further supporting the hypothesis of a developmental link.







Similar content being viewed by others
References
Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S (2014) Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer Int. J Endocrinol 2014:790834
Lloyd RV, Osamura RY, Klöppel G, Rosai J (2017) WHO classification of tumours of endocrine organs, 4th Edition. IARC, Lyon
Albores-Saavedra J, Hernandez M, Sanchez-Sosa S, Simpson K, Angeles A, Henson DE (2007) Histologic variants of papillary and follicular carcinomas associated with anaplastic spindle and giant cell carcinomas of the thyroid: an analysis of rhabdoid and thyroglobulin inclusions. Am J Surg Pathol 31:729–736
Carcangiu ML, Steeper T, Zampi G, Rosai J (1985) Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol 83:135–158
Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA (1990) Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer 66:321–330
Rapkiewicz A, Roses D, Goldenberg A, Levine P, Bannan M, Simsir A (2009) Encapsulated anaplastic thyroid carcinoma transformed from follicular carcinoma: a case report. Acta Cytol 53:332–336
Ibanez ML, Russell WO, Albores-Saavedra J, Lampertico P, White EC, Clark RL (1966) Thyroid carcinoma—biologic behavior and mortality. Postmortem findings in 42 cases, including 27 in which the disease was fatal. Cancer 19:1039–1052
Nishiyama RH, Dunn EL, Thompson NW (1972) Anaplastic spindle-cell and giant-cell tumors of the thyroid gland. Cancer 30:113–127
Aldinger KA, Samaan NA, Ibanez M, Hill CS Jr (1978) Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer 41:2267–2275
Aratake Y, Nomura H, Kotani T, Marutsuka K, Kobayashi K, Kuma K, Miyauchi A, Okayama A, Tamura K (2006) Coexistent anaplastic and differentiated thyroid carcinoma: an immunohistochemical study. Am J Clin Pathol 125:399–406
Bronner MP, LiVolsi VA (1991) Spindle cell squamous carcinoma of the thyroid: an unusual anaplastic tumor associated with tall cell papillary cancer. Mod Pathol 4:637–643
Camargo R, Limbert E, Gillam M, Henriques MM, Fernandes C, Catarino AL, Soares J, Alves VA, Kopp P, Medeiros-Neto G (2001) Aggressive metastatic follicular thyroid carcinoma with anaplastic transformation arising from a long-standing goiter in a patient with Pendred's syndrome. Thyroid 11:981–988
Capdevila J, Mayor R, Mancuso FM, Iglesias C, Caratu G, Matos I, Zafon C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Alvarez CV, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J (2018) Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol 29:1454–1460
Choi JY, Hwang BH, Jung KC, Min HS, Koo Do H, Youn YK, Lee KE (2013) Clinical significance of microscopic anaplastic focus in papillary thyroid carcinoma. Surgery 154:106–110
Deeken-Draisey A, Yang GY, Gao J, Alexiev BA (2018) Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol 82:140–148
Demeter JG, De Jong SA, Lawrence AM, Paloyan E (1991) Anaplastic thyroid carcinoma: risk factors and outcome. Surgery 110:956–961 discussion 961-953
Dong W, Nicolson NG, Choi J, Barbieri AL, Kunstman JW, Abou Azar S, Knight J, Bilguvar K, Mane SM, Lifton RP, Korah R, Carling T (2018) Clonal evolution analysis of paired anaplastic and well-differentiated thyroid carcinomas reveals shared common ancestor. Genes Chromosom Cancer 57:645–652
Fortson JK, Durden FL Jr, Patel V, Darkeh A (2004) The coexistence of anaplastic and papillary carcinomas of the thyroid: a case presentation and literature review. Am Surg 70:1116–1119
Galera-Davidson H, Bibbo M, Dytch HE, Gonzalez-Campora R, Fernandez A, Wied GL (1987) Nuclear DNA in anaplastic thyroid carcinoma with a differentiated component. Histopathology 11:715–722
Gopal PP, Montone KT, Baloch Z, Tuluc M, Livolsi V (2011) The variable presentations of anaplastic spindle cell squamous carcinoma associated with tall cell variant of papillary thyroid carcinoma. Thyroid 21:493–499
Hunt JL, Tometsko M, LiVolsi VA, Swalsky P, Finkelstein SD, Barnes EL (2003) Molecular evidence of anaplastic transformation in coexisting well-differentiated and anaplastic carcinomas of the thyroid. Am J Surg Pathol 27:1559–1564
Kleer CG, Giordano TJ, Merino MJ (2000) Squamous cell carcinoma of the thyroid: an aggressive tumor associated with tall cell variant of papillary thyroid carcinoma. Mod Pathol 13:742–746
Lai WA, Hang JF, Liu CY, Bai Y, Liu Z, Gu H, Hong S, Pyo JY, Jung CK, Kakudo K, Bychkov A (2020) PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch 476:431–437
Lam KY, Lo CY, Chan KW, Wan KY (2000) Insular and anaplastic carcinoma of the thyroid: a 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg 231:329–338
Lam KY, Lo CY, Wei WI (2005) Warthin tumor-like variant of papillary thyroid carcinoma: a case with dedifferentiation (anaplastic changes) and aggressive biological behavior. Endocr Pathol 16:83–89
Ma B, Xu W, Wei W, Wen D, Lu Z, Yang S, Chen T, Wang Y, Wang Y, Ji Q (2018) Clinicopathological and survival outcomes of well-differentiated thyroid carcinoma undergoing dedifferentiation: a retrospective study from FUSCC. Int J Endocrinol 2018:2383715
Mai DD, Mai KT, Shamji FM (2001) Fine needle aspiration biopsy of anaplastic thyroid carcinoma developing from a Hürthle cell tumor: a case report. Acta Cytol 45:761–764
Matias-Guiu X, Cuatrecasas M, Musulen E, Prat J (1994) p53 expression in anaplastic carcinomas arising from thyroid papillary carcinomas. J Clin Pathol 47:337–339
Matias-Guiu X, Villanueva A, Cuatrecasas M, Capella G, De Leiva A, Prat J (1996) p53 in a thyroid follicular carcinoma with foci of poorly differentiated and anaplastic carcinoma. Pathol Res Pract 192:1242–1249 discussion 1250-1241
McDonald RJ, Wu SY, Jensen JL, Parker LN, Lyons KP, Moran EM, Blahd WH (1991) Malignant transformation of a Hürthle cell tumor: case report and survey of the literature. J Nucl Med 32:1266–1269
McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR (2001) Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 130:1028–1034
Mochizuki K, Kondo T, Nakazawa T, Iwashina M, Kawasaki T, Nakamura N, Yamane T, Murata S, Ito K, Kameyama K, Kobayashi M, Katoh R (2010) RET rearrangements and BRAF mutation in undifferentiated thyroid carcinomas having papillary carcinoma components. Histopathology 57:444–450
Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88:5399–5404
Oishi N, Kondo T, Ebina A, Sato Y, Akaishi J, Hino R, Yamamoto N, Mochizuki K, Nakazawa T, Yokomichi H, Ito K, Ishikawa Y, Katoh R (2017) Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: identification of TERT mutation as an independent risk factor for transformation. Mod Pathol 30:1527–1537
Okon K, Wierzchowski W, Jablonska E, Wojcik P, Steczko A (2003) Anaplastic, sarcomatoid carcinoma of the thyroid originating from a Hürthle cell tumor. Pol J Pathol 54:277–281
Park JH, Kwon HJ, Park CS, Hong S (2014) Anaplastic transformation of papillary thyroid carcinoma in a young man: a case study with immunohistochemical and BRAF analysis. Korean J Pathol 48:234–240
Patten DK, Ahmed A, Greaves O, Dina R, Flora R, Tolley N (2017) Anaplastic spindle cell squamous carcinoma arising from tall cell variant papillary carcinoma of the thyroid gland: a case report and review of the literature. Case Rep Endocrinol 2017:4581626
Rosai J, De Lellis RA, Carcangiu ML, Frable WJ, Tallini G (2014) Undifferentiated (anaplastic) thyroid carcinoma. In: Tumors of the thyroid and parathyroid glands. AFIP atlas of tumor pathology. Series 4, Silver Spring, Maryland (USA), pp. 177–198
Saunders CA, Nayar R (1999) Anaplastic spindle-cell squamous carcinoma arising in association with tall-cell papillary cancer of the thyroid: a potential pitfall. Diagn Cytopathol 21:413–418
Spires JR, Schwartz MR, Miller RH (1988) Anaplastic thyroid carcinoma. Association with differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg 114:40–44
Stucchi CM, Vaccaro V, Magherini A, Di Gregorio C, Greco G, Livolsi VA, Papi G (2007) Hürthle cell follicular carcinoma of the thyroid gland presenting with diffuse meningeal carcinomatosis and evolving to anaplastic carcinoma. J Clin Pathol 60:831–832
Takano T, Ito Y, Hirokawa M, Yoshida H, Miyauchi A (2007) BRAF V600E mutation in anaplastic thyroid carcinomas and their accompanying differentiated carcinomas. Br J Cancer 96:1549–1553
Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP (1995) Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck 17:41–47 discussion 47–48
Wang HM, Huang YW, Huang JS, Wang CH, Kok VC, Hung CM, Chen HM, Tzen CY (2007) Anaplastic carcinoma of the thyroid arising more often from follicular carcinoma than papillary carcinoma. Ann Surg Oncol 14:3011–3018
Wiseman SM, Loree TR, Hicks WL Jr, Rigual NR, Winston JS, Tan D, Anderson GR, Stoler DL (2003) Anaplastic thyroid cancer evolved from papillary carcinoma: demonstration of anaplastic transformation by means of the inter-simple sequence repeat polymerase chain reaction. Arch Otolaryngol Head Neck Surg 129:96–100
Chen TY, Lorch JH, Wong KS, Barletta JA (2020) Histologic features of BRAF V600E-mutant anaplastic thyroid carcinoma. Histopathology. https://doi.org/10.1111/his.14144.
Wong KS, Lorch JH, Alexander EK, Marqusee E, Cho NL, Nehs MA, Doherty GM, Barletta JA (2020) Histopathologic features and clinical outcome of anaplastic thyroid carcinoma with a minor anaplastic component. Endocr Pathol. https://doi.org/10.1007/s12022-020-09627-0
Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, Gunn GB, Lu C, Ferrarotto R, Lai SY, Cabanillas ME (2017) Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid 27:672–681
Tiedje V, Ting S, Herold T, Synoracki S, Latteyer S, Moeller LC, Zwanziger D, Stuschke M, Fuehrer D, Schmid KW (2017) NGS based identification of mutational hotspots for targeted therapy in anaplastic thyroid carcinoma. Oncotarget 8:42613–42620
Bonhomme B, Godbert Y, Perot G, Al Ghuzlan A, Bardet S, Belleannee G, Criniere L, Do Cao C, Fouilloux G, Guyetant S, Kelly A, Leboulleux S, Buffet C, Leteurtre E, Michels JJ, Tissier F, Toubert ME, Wassef M, Pinard C, Hostein I, Soubeyran I (2017) Molecular pathology of anaplastic thyroid carcinomas: a retrospective study of 144 cases. Thyroid 27:682–692
Hirokawa M, Sugitani I, Kakudo K, Sakamoto A, Higashiyama T, Sugino K, Toda K, Ogasawara S, Yoshimoto S, Hasegawa Y, Imai T, Onoda N, Orita Y, Kammori M, Fujimori K, Yamada H (2016) Histopathological analysis of anaplastic thyroid carcinoma cases with long-term survival: a report from the Anaplastic Thyroid Carcinoma Research Consortium of Japan. Endocr J 63:441–447
Van der Laan B, Freeman JL, Tsanq RW, Asa SL (1993) The association of well-differentiated thyroid carcinoma with insular or anaplastic thyroid carcinoma; evidence for dedifferentiation in tumor progression. Endocr Pathol 4:215–221
Kuhn E, Ragazzi M, Ciarrocchi A, Torricelli F, de Biase D, Zanetti E, Bisagni A, Corrado S, Uccella S, La Rosa S, Bongiovanni M, Losito S, Piana S (2019) Angiosarcoma and anaplastic carcinoma of the thyroid are two distinct entities: a morphologic, immunohistochemical, and genetic study. Mod Pathol 32:787–798
Clinton LK, Miyazaki K, Ayabe A, Davis J, Tauchi-Nishi P, Shimizu D (2015) The LAST guidelines in clinical practice: implementing recommendations for p16 use. Am J Clin Pathol 144:844–849
de Biase D, Acquaviva G, Visani M, Sanza V, Argento CM, De Leo A, Maloberti T, Pession A, Tallini G (2020) Molecular diagnostic of solid tumor using a next generation sequencing custom-designed multi-gene panel. Diagnostics (Basel) 10:25010
de Biase D, Torricelli F, Ragazzi M, Donati B, Kuhn E, Visani M, Acquaviva G, Pession A, Tallini G, Piana S, Ciarrocchi A (2018) Not the same thing: metastatic PTCs have a different background than ATCs. Endocr Connect 7:1370–1379
Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, Tuttle RM, Sherman E, Gill AJ, Ghossein R (2020) Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid. https://doi.org/10.1089/thy.2020.0086
Asioli S, Erickson LA, Righi A, Lloyd RV (2013) Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Hum Pathol 44:320–328
Goffredo P, Roman SA, Sosa JA (2013) Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer 119:504–511
Lam AK (2020) Squamous cell carcinoma of thyroid: a unique type of cancer in World Health Organization classification. Endocr Relat Cancer 27:R177–R192
Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA (2016) Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066
Gandolfi G, Ragazzi M, de Biase D, Visani M, Zanetti E, Torricelli F, Sancisi V, Gugnoni M, Manzotti G, Braglia L, Cavuto S, Merlo DF, Tallini G, Frasoldati A, Piana S, Ciarrocchi A (2018) Genome-wide profiling identifies the THYT1 signature as a distinctive feature of widely metastatic papillary thyroid carcinomas. Oncotarget 9:1813–1825
de Biase D, Gandolfi G, Ragazzi M, Eszlinger M, Sancisi V, Gugnoni M, Visani M, Pession A, Casadei G, Durante C, Costante G, Bruno R, Torlontano M, Paschke R, Filetti S, Piana S, Frasoldati A, Tallini G, Ciarrocchi A (2015) TERT promoter mutations in papillary thyroid microcarcinomas. Thyroid 25:1013–1019
Cameselle-Teijeiro JM, Rodriguez-Perez I, Celestino R, Eloy C, Piso-Neira M, Abdulkader-Nallib I, Soares P, Sobrinho-Simoes M (2017) Hobnail variant of papillary thyroid carcinoma: clinicopathologic and molecular evidence of progression to undifferentiated carcinoma in 2 cases. Am J Surg Pathol 41:854–860
Contributions
Investigation: MR, SP, FT, BD, EK, DG, AF, and DdB. Data curation: MR, SP, FT, BD, AB, and EZ. Writing—original draft preparation: MR and SP. Writing—review and editing: AC. Supervision: GT. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Italian Ministry of Health, grant number RF-2016-02364167.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Institutional Review Board of the Azienda USL-IRCCS, Reggio Emilia, Italy (protocol no. 71141–2017).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Ragazzi, M., Torricelli, F., Donati, B. et al. Coexisting well-differentiated and anaplastic thyroid carcinoma in the same primary resection specimen: immunophenotypic and genetic comparison of the two components in a consecutive series of 13 cases and a review of the literature. Virchows Arch 478, 265–281 (2021). https://doi.org/10.1007/s00428-020-02891-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02891-9